Health
Ohio woman, the first person to receive a breast cancer vaccine in trial, awaits results: ‘Very excited’

Whereas a breast most cancers vaccine just isn’t but accepted for widespread use, there are trials underway — together with one at Cleveland Clinic, the place 46-year-old Jennifer Davis of Ohio was the primary individual to get the shot in 2021.
The vaccine had been in growth at Cleveland Clinic for greater than 20 years earlier than it lastly reached the human trial section. Now, researchers are hopeful it could possibly be out there to sure most cancers survivors inside a couple of years.
In an interview with Fox Information Digital, Davis mentioned the vaccine has introduced her peace of thoughts that the illness could possibly be behind her for good.
OHIO WOMAN PUSHES PAST BREAST CANCER, WON’T LET DIAGNOSIS SLOW HER DOWN
“All of it fell into place and labored out completely,” she mentioned — although her journey just isn’t over but.
A very long time coming
The breast most cancers vaccine has been licensed to Anixa Biosciences, which is working with Cleveland Clinic on the rollout. Fox Information Digital spoke with Dr. Amit Kumar, CEO of Anixa, concerning the prolonged journey to convey the vaccine to trial.
Kumar defined that Dr. Vince Tuohy, an immunologist on the Cleveland Clinic, invented the vaccine that is presently being examined.
Jennifer Davis of Ohio is pictured right here with images of herself throughout her most cancers journey. She was the primary individual to obtain a breast most cancers vaccine in 2021 at Cleveland Clinic. Now, she’s awaiting the outcomes. (Jennifer Davis)
“Vince ran the analysis group that performed the analysis on this vaccine for twenty years,” Kumar mentioned. “He was an excellent scientist and we turned good associates as we labored collectively. Sadly, he handed away a couple of weeks in the past on the age of 74.”
Now, Tuohy’s fellow researchers — together with Dr. G. Thomas Budd and Dr. Justin Johnson — are persevering with work on the vaccine, in collaboration with Kumar and his group.
Ohio most cancers survivor was first to be vaccinated
Davis is a nurse who has three grownup youngsters. She was initially recognized with triple-negative breast most cancers in 2018.
Triple-negative is a extra aggressive kind of breast most cancers that doesn’t have any of the three frequent “receptors” within the cells, which implies it doesn’t reply to the hormonal therapies which are sometimes used to battle the illness.
About 15% of all breast cancers fall into this class.
Triple-negative breast most cancers is extra aggressive and more durable to deal with.
Davis had a rigorous spherical of therapies that included chemotherapy, surgical procedure and 26 rounds of radiation. Whereas the remedy labored and she or he was pronounced cancer-free, she was nonetheless involved.
“Triple-negative most cancers is so aggressive and recurrence is basically, actually excessive — the prognosis just isn’t the best,” she informed Fox Information Digital.
“And there was nothing I may take following remedy. As soon as remedy is over, there isn’t any capsule or something that offers you that peace of thoughts that it is not going to return again.”

Davis, pictured with a photograph of herself receiving the vaccine, mentioned the shot has given her peace of thoughts and lowered her worries about her breast most cancers coming again. (Jennifer Davis)
When Davis heard concerning the vaccine trial at Cleveland Clinic, she utilized and was thrilled to get picked.
“There have been very particular tips and a variety of testing I needed to undergo,” she mentioned.
“I used to be so near not having the ability to get it,” she additionally mentioned, including that “all of it fell into place and labored out completely.”
NEW BREAST CANCER GENE CAN PREDICT LIKELIHOOD OF HEREDITARY DISEASE, STUDY FINDS
Davis obtained her first dose of the vaccine on Oct. 19, 2021. After that, she obtained two further doses spaced two weeks aside.
Then started the lengthy wait to seek out out whether or not the photographs labored.
Hoping for excellent news
The vaccine has been administered to 14 sufferers thus far, Dr. Kumar mentioned.
Subsequent week, on the annual assembly of the American Affiliation of Most cancers Analysis, the analysis group will current the scientific information for the primary group of sufferers.
“The info is wanting superb thus far,” the physician mentioned.

Earlier than getting the vaccine, Davis (pictured together with her husband) went by means of grueling therapies that included chemotherapy, surgical procedures and 26 rounds of radiation. Of the breast most cancers vaccine she obtained through the trial interval, she mentioned, “There have been very particular tips and a variety of testing I needed to undergo.” Now, she’s awaiting the outcomes. (Jennifer Davis)
As Davis defined, she had lab work carried out earlier than and after every vaccine dose.
She has not but seen the outcomes of these exams — so she’s wanting ahead to the presentation subsequent week.
“I’m very, very excited,” she mentioned. “What I am ready to seek out out, what all people is seeking to see, is whether or not I constructed up an immune response to the breast most cancers.”
“Each lady on the earth may probably be a candidate for this vaccine.”
If the outcomes are as constructive as she hopes, Davis appears to be like ahead to a time when extra girls could have entry to the vaccine — not simply those that have already been handled for triple-negative most cancers, like her, but additionally wholesome girls who need to forestall most cancers from growing within the first place.
“On the grander scale, this might remove triple-negative breast most cancers,” she mentioned.
“If that piece of the puzzle could possibly be utterly eliminated and we by no means needed to fear about it once more, that may be wonderful.”
Trying forward with optimism
Immediately, Davis is simply six months away from being cancer-free for 5 years, a milestone she doesn’t take flippantly.
She mentioned receiving the vaccine has given her a way of hope which may not in any other case have been as straightforward to return by.
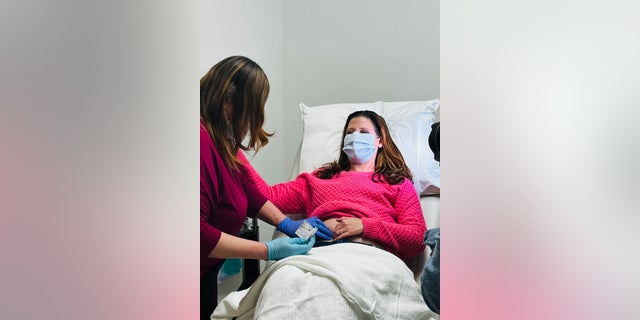
Davis (proven right here with masks on) appears to be like ahead to a time when extra girls could have entry to the vaccine — not simply those that have already been handled for triple-negative breast most cancers, but additionally wholesome girls who need to forestall most cancers from growing within the first place. (Cleveland Clinic)
“It was that for each little headache, I’d assume I may need a mind tumor, and if my arm began to harm, I’d assume I had bone most cancers,” she mentioned. “There was that fixed fear that one thing has was one thing else. However after the vaccine, although I do not know the outcomes of it but, I don’t have that fear anymore, which has been fantastic.”
Kumar mentioned he’s additionally hopeful the vaccine will remove that anxiousness in all girls.
“I’d love to have the ability to give this vaccine to my daughters to cut back their danger.”
“Within the U.S., there are 3.6 million girls who’re breast most cancers survivors, they usually get up each morning apprehensive that their most cancers goes to return again,” he mentioned.
“We’d love to have the ability to give all of these girls a shot so that they don’t have to fret about most cancers recurrence.”
FDA ISSUES NEW MAMMOGRAM REGULATIONS AIMED AT FURTHER BREAST CANCER PREVENTION
That is particularly essential for triple-negative most cancers, which is often way more aggressive if it comes again, he mentioned.
The physician foresees the vaccine’s eventual availability to all girls, even these with out prior breast most cancers diagnoses.
The breast most cancers vaccine was in growth at Cleveland Clinic for greater than 20 years earlier than lastly reaching the human trial section. (iStock)
“As soon as we have accomplished all the suitable medical research, each lady on the earth may probably be a candidate for this vaccine,” he mentioned.
“We consider the vaccine shall be out there inside 4 to 5 years for recurrence prevention after which for main prevention a couple of years after that,” the physician added.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
“Recurrence prevention” focuses on folks like Davis, who’ve beforehand had breast most cancers and face a excessive danger of recurrence, Kumar defined.
“Major prevention” refers back to the vaccination of ladies who’ve by no means had most cancers to assist guarantee they do not get it.
“I’ve two daughters, and our household has a historical past of breast most cancers,” Kumar mentioned. “I’d love to have the ability to give this vaccine to my daughters to cut back their danger.”
Breast most cancers is the world’s most typical kind of most cancers, with 2.3 million girls recognized in 2020, per the World Well being Group.

Health
Treating Other Diseases With Ozempic? Experts Weigh In | Woman's World

Sign Up
Create a free account to access exclusive content, play games, solve puzzles, test your pop-culture knowledge and receive special offers.
Already have an account? Login
Use left and right arrow keys to navigate between menu items.
Use escape to exit the menu.
Health
FDA bans red food dye due to potential cancer risk

FDA looks to ban red food dye
Celebrity fitness trainer Jillian Michaels joins ‘Hannity’ to discuss the possibility of the FDA banning red food dye.
The U.S. Food and Drug Administration (FDA) has officially banned red dye — called Red 3, or Erythrosine — from foods, dietary supplements and ingested medicines, as reported by the Associated Press on Wednesday.
Food manufacturers must remove the dye from their products by January 2027, while drug manufacturers will have until January 2028 to do so, AP stated.
Any foods imported into the U.S. from other countries will also be subject to the new regulation.
RED FOOD DYE COULD SOON BE BANNED AS FDA REVIEWS PETITION
“The FDA is taking action that will remove the authorization for the use of FD&C Red No. 3 in food and ingested drugs,” said Jim Jones, the FDA’s deputy commissioner for human foods, in a statement.
The U.S. Food and Drug Administration has officially banned red dye — called Red 3, or Erythrosine — from foods, dietary supplements and ingested medicines (iStock)
“Evidence shows cancer in laboratory male rats exposed to high levels of FD&C Red No.3,” he continued. “Importantly, the way that FD&C Red No. 3 causes cancer in male rats does not occur in humans.”
The synthetic dye, which is made from petroleum, is used as a color additive in food and ingested drugs to give them a “bright cherry-red color,” according to an online statement from the FDA.

Food manufacturers must remove the dye from their products by January 2027, while drug manufacturers will have until January 2028 to do so. (iStock)
The petition to ban the dye cited the Delaney Clause, which states that the agency cannot classify a color additive as safe if it has been found to induce cancer in humans or animals.
The dye was removed from cosmetics nearly 35 years ago due to potential cancer risk.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
“This is a welcome, but long overdue, action from the FDA: removing the unsustainable double standard in which Red 3 was banned from lipstick but permitted in candy,” said Dr. Peter Lurie, director of the group Center for Science in the Public Interest, which led the petition effort, as reported by AP.

Nearly 3,000 foods are shown to contain Red No. 3, according to Food Scores, a database of foods compiled by the Environmental Working Group. (iStock)
Dr. Marc Siegel, clinical professor of medicine at NYU Langone Health and Fox News senior medical analyst, applauded the FDA’s ban.
“It was a long time coming,” he told Fox News Digital. “It’s been more than 30 years since it was banned from cosmetics in the U.S. due to evidence that it is carcinogenic in high doses in lab rats. There needs to be a consistency between what we put on our skin and what we put into our mouths.”
“There needs to be a consistency between what we put on our skin and what we put into our mouths.”
Siegel said he believes the FDA’s decision could be tied to the incoming new head of the Department of Health and Human Services, Robert F. Kennedy Jr.
“They knew it would have happened anyway under RFK Jr.,” he said. “It is already banned or severely restricted in Australia, Japan and the European Union.”

The food additive also “drew kids in” to a diet of empty calories and ultraprocessed foods, one doctor stated. (iStock)
The food additive also “drew kids in” to a diet of empty calories and ultraprocessed foods, Siegel added.
“It has also been linked to behavioral issues in children, including ADHD.”
Nearly 3,000 foods are shown to contain Red No. 3, according to Food Scores, a database of foods compiled by the Environmental Working Group.
For more Health articles, visit www.foxnews.com/health
The National Confectioners Association provided the below statement to Fox News Digital.
“Food safety is the number one priority for U.S. confectionery companies, and we will continue to follow and comply with FDA’s guidance and safety standards.”
The petition to remove Red No. 3 from foods, supplements and medications was presented in 2022 by the Center for Science in the Public Interest and 23 other organizations and scientists.
Health
How Yvette Nicole Brown Lost Weight and Got Her Diabetes Under Control

Sign Up
Create a free account to access exclusive content, play games, solve puzzles, test your pop-culture knowledge and receive special offers.
Already have an account? Login
Use left and right arrow keys to navigate between menu items.
Use escape to exit the menu.
-
/cdn.vox-cdn.com/uploads/chorus_asset/file/25822586/STK169_ZUCKERBERG_MAGA_STKS491_CVIRGINIA_A.jpg)
/cdn.vox-cdn.com/uploads/chorus_asset/file/25822586/STK169_ZUCKERBERG_MAGA_STKS491_CVIRGINIA_A.jpg) Technology1 week ago
Technology1 week agoMeta is highlighting a splintering global approach to online speech
-

 Science7 days ago
Science7 days agoMetro will offer free rides in L.A. through Sunday due to fires
-
/cdn.vox-cdn.com/uploads/chorus_asset/file/23935558/acastro_STK103__01.jpg)
/cdn.vox-cdn.com/uploads/chorus_asset/file/23935558/acastro_STK103__01.jpg) Technology6 days ago
Technology6 days agoAmazon Prime will shut down its clothing try-on program
-

 News1 week ago
News1 week agoMapping the Damage From the Palisades Fire
-

 News1 week ago
News1 week agoMourners Defy Subfreezing Temperatures to Honor Jimmy Carter at the Capitol
-
/cdn.vox-cdn.com/uploads/chorus_asset/file/25826211/lorealcellbioprint.jpg)
/cdn.vox-cdn.com/uploads/chorus_asset/file/25826211/lorealcellbioprint.jpg) Technology6 days ago
Technology6 days agoL’Oréal’s new skincare gadget told me I should try retinol
-
/cdn.vox-cdn.com/uploads/chorus_asset/file/25832751/2192581677.jpg)
/cdn.vox-cdn.com/uploads/chorus_asset/file/25832751/2192581677.jpg) Technology3 days ago
Technology3 days agoSuper Bowl LIX will stream for free on Tubi
-

 Business4 days ago
Business4 days agoWhy TikTok Users Are Downloading ‘Red Note,’ the Chinese App















