Science
Column: This company made billions by surprise-billing helpless ER patients. Then justice arrived

Some businesses deserve sympathy when they land in bankruptcy. Then there’s Envision.
The Tennessee company prospered as a provider of medical staff to hospitals around the country. It concentrated on emergency physicians, anesthesiologists and radiologists for a simple reason: Their patients typically had no ability to pick and choose among these doctors when they need care.
Waiting to be seen at the emergency room or already laid out on an operating table, the patients weren’t in a position to ask whether the doctor was in their insurance plan’s network.
These higher payment rates [are] caused not by supply or demand, but rather by the ability to “ambush” the patient.”
— Zack Cooper et al, Yale University
They might not even know they were seen by out-of-network doctors until they received a bill for their services — a surprise invoice for hundreds or even thousands of dollars.
Envision’s “secret sauce was to pile medical debt on people with emergencies,” says Eileen Appelbaum, co-director of the Center for Economic and Policy Research, a nonprofit Washington think tank. “That was purely taking advantage of people at their most vulnerable time.”
Envision stands as a case study in the destructive incursion of the profit motive — actually, the profiteering motive — in American healthcare.
Doctors themselves aren’t the problem — they’re disadvantaged themselves by financial pressures imposed by profit-seeking firms, which often require them to see more patients in a day and work without the best equipment.
Surprise billing — sometimes known as “balance billing” — by Envision and other firms like it elicited so much public outrage that Congress was finally moved to do something about the practice. In 2020 it enacted the No Surprises Act, which went into effect on Jan. 1, 2022.
Envision wasn’t alone in sticking patients with unexpected bills; another staffing firm, TeamHealth, is also struggling with the ramifications of the No Surprises Act.
The act prohibited out-of-network providers who were not chosen by a patient from charging the patient more than the in-network reimbursement fee set by the patient’s health plan. It forbade insurers to reject outright a patient’s claim for service from an out-of-network doctor.
Federal officials estimated that the law would apply to about 10 million unexpected bills a year. That figure didn’t include beneficiaries of another provision, which outlawed the very sleazy practice by some big insurers of rejecting an emergency-room claim because a patient’s condition didn’t turn out to be a true emergency.
The No Surprises Act blew a sizable hole in Envision’s profit-and-loss statement — part of “a whiplash-inducing onslaught of obstacles and complications” facing the firm’s management, its bankruptcy filing stated.
Among other elements of the “onslaught” the firm cited was the COVID pandemic, which reduced non-emergency hospital visits by as much as 70% because patients deferred elective surgeries; an increase in salaries for professionals as the pandemic prompted older clinicians to retire; and a billing backlash by its largest payer, UnitedHealth Group.
Envision, which provides staff for more than 500 facilities in 45 states, said in its bankruptcy filing that the pandemic cost it some $795 million in operating revenue (technically, earnings before interest, taxes, depreciation and amortization, or EBITDA) in 2020 and 2021.
UnitedHealth’s “uniquely aggressive” pushback on reimbursements, Envision said, cost it more than $400 million in EBITDA over the last five years. Envision says that if UnitedHealth merely paid what Envision claims it owes, it would not have had to file for bankruptcy. Overall, the company’s EBITDA went from about $1 billion before the pandemic to about $250 million last year.
In any case, the No Surprises Act was the factor that hit Envision’s business model below the water line.
It’s worthwhile, then, to take a closer look at what this firm and its owner, the private equity firm Kohlberg Kravis Roberts & Co., have been up to, and why we should celebrate the modest change in the American healthcare system represented by the act. (TeamHealth is owned by another private equity firm, Blackstone.)
Private equity firms acquire businesses typically through leveraged buyouts, in which the acquisition is financed largely through borrowings to be paid back out of the acquired business’ revenues. Generally, their goal is to cash out by selling the business or taking it public within about five years.
The firms first showed interest in healthcare businesses in the 1990s, initially focusing on nursing homes and hospitals because of their reliable cash flows, as a Brookings Institution study outlined in 2021. By 2010, the private equity firms had moved on to urgent care clinics, ambulance services, and emergency departments and hospital services such as anesthesiology and radiology “that could utilize surprise out-of-network billing,” Brookings found.
According to a 2018 Yale University study that heavily influenced the legislative movement in Congress, it wasn’t unusual for emergency doctors to end their network contract with insurers. On average, the Yale researchers reported, out-of-network physicians charged more than twice the going in-network insurance reimbursements and more than six times the standard reimbursements from Medicare.
The study in effect pointed the finger at the physician staffing firms, Envision (known at the time as EmHealth) and TeamHealth. “Both firms profit from the fact that out-of-network physicians working in in-network hospitals cannot be avoided by patients.” The runup in charges, the researchers wrote, “undercuts the functioning of healthcare labor markets, exposes patients to significant financial risk, and reduces social welfare.”
“These higher payment rates” charged by out-of-network physicians, the Yale study said, are “caused not by supply or demand, but rather by the ability to ‘ambush’ the patient.” The practice raised the cost of healthcare generally.
The Yale researchers made passing mention of another player in this process: the health insurance industry, which has an interest in paying providers as little as it can get away with. (UnitedHealth, as it happens, provided the researchers with the data they used for their study, leading to accusations that the study favored the health plans’ point of view; the researchers said UnitedHealth had no influence on their findings.)
Insurers reacted to the charges from out-of-network doctors by paying only a portion of their bills. That prompted doctors to try to obtain the balance by billing the patients. The surprise billing scandal was born.
Envision says it started to phase out surprise or balance billing in 2020, even before the No Surprises Act was passed and signed — though congressional action against the practice was in the wind long before that.
It says its real problem is that regulations implementing the act have put health insurers in the driver’s seat in setting reimbursements. Provider firms have to go to arbitration to settle their differences, but that’s resulted in a logjam of arbitration cases and a backup of unpaid claims.
Private equity investors had long had their eyes on Envision, which was founded in 1992. The firm oscillated between private and public ownership, as a succession of investors sought to squeeze it for profits.
The process started in 2005, when Onyx Capital acquired it through a leveraged buyout and took it public the same year. The investment firm Clayton, Dubilier & Rice acquired it in 2011 for $3.2 billion and staged an initial public offering two years later.
Soon after that, EmHealth merged with Amsurg, a physician staffing service for ambulatory surgical centers — that is, those unaffiliated with hospitals. The result was Envision, which became the largest physician staffing firm in the country.
Kohlberg Kravis Roberts entered the scene in 2018 with a $9.9-billion leveraged buyout, in which the firm and its partners invested $3.5 billion. That stake was wiped out in the bankruptcy, which followed lengthy and extremely complex negotiations with creditors that included Newport Beach-based Pimco.
The prearranged bankruptcy will allow both Amsurg and Envision to continue operating under the ownership of their creditors, who will take over once the latter emerges from bankruptcy in the fall. Among the new owners will be Blackstone, giving the big private equity firm a foothold in the two biggest staffing companies, Envision and TeamHealth.
The No Surprises Act did little to remedy the dysfunction of American healthcare. If anything, it underscored the insanity of a system that locks providers and payers in never-ending conflict over who owes what to whom and for which services.
Envision and UnitedHealth waged this war for years. The two parties let their last network contract expire in January 2021, after which they continued to snipe at each other over reimbursements until last September, when they served each other with lawsuits.
Envision says UnitedHealth has consistently low-balled its reimbursements ever since the contract expiration — in fact, it says Envision allowed the contract to lapse because it wouldn’t accept UnitedHealth’s “unconscionable take-it-or-leave-it reimbursement offer.”
The insurer, for its part, says Envision has “systematically” upcoded its reimbursement claims. The allegation is that Envision has submitted claims for more elaborate patient treatments — which warrant higher reimbursements — than the patients needed or received at the ER.
(Envision won a $91-million arbitation award from UnitedHealth in March, but that dispute concerned reimbursements owed in 2017 and 2018, while the firms’ network contract was in effect.)
It’s tempting to declare a plague on both their houses, to quote “Romeo and Juliet,” but the problem is systemic. It’s grounded in “the tension between profit maximization by firms that own health care providers, and the best care possible for patients that providers are obligated to provide,” Appelbaum has observed.
The No Surprises Act addressed one symptom of that tension, the practice of sticking unwary patients with unexpected bills. But it didn’t lay a finger on the underlying disease. Americans spend vastly more on medical care than residents of any other developed country, and have worse health outcomes.
One reason is the endless, costly dickering among middlemen like Envision and UnitedHealth to make sure they get their share of the bucks sloshing around in the system. Every segment of the healthcare industry also spends heavily on lobbyists in Washington to ensure they don’t get shut out of the party.
Who doesn’t have lobbyists? The patients. The No Surprises Act is one of the very few victories they’ve ever notched in this battle, and they shouldn’t expect to see many more.

Science
Solar storm heading to Earth could disrupt communications and bring northern lights to California

A different kind of storm could complicate this weekend’s plans.
For the first time since January 2005, the U.S. National Oceanic and Atmospheric Administration has issued a severe geomagnetic storm watch for Friday evening.
The category G4 watch from NOAA’s Space Weather Prediction Center signals the possibility that a concentration of energy flaring from the Sun could disturb our planet’s electromagnetic field once it reaches Earth.
A geomagnetic storm of this size could disrupt communications, like the 2003 event that caused blackouts in Sweden and damaged South Africa’s power grid.
More promisingly, super-charged collisions of solar energy into the gas of our atmosphere creates the dazzling phenomena of aurora borealis, or the northern lights. Typically confined to polar regions, the colorful display could be visible this weekend as far south as Northern California.
“We have a very rare event on our hands,” space weather forecaster Shawn Dahl of SWPC said during a news conference Friday morning.
A geomagnetic storm happens when energy from solar wind — the high-speed current of atomic particles the sun is constantly flinging into space — is transferred into the electromagnetic field that surrounds Earth.
The winds of a Category 5 hurricane on our puny little planet can exceed 150 miles per hour. In contrast, solar wind averages about 870,000 miles per hour all day, every day according to NASA.
Earth’s electromagnetic field deflects the majority of these particles, save for those interactions in the polar areas that produce the northern lights. But unusually intense or concentrated eruptions of energy on the surface of the Sun can disrupt that equilibrium, causing geomagnetic storms like the one potentially headed our way.
On Wednesday morning, astronomers noted a series of solar flares and coronal mass ejections — essentially, giant explosions of energy — emanating from a massive sunspot more than 15 times the diameter of Earth.
The Space Weather Prediction Center has observed seven coronal mass ejections, or CMEs, heading in Earth’s direction, said Mike Bettwy, operations chief at SWPC.
“Based on the data we have, all seven of these are going to be spewing that energy toward us,” Bettwy said.
The energy in these various eruptions is expected to merge and reach the Earth’s magnetic field late Friday or early Saturday.
“Our level of confidence is high that we will have an arrival of these CMEs as early as this evening,” Dahl said. The precise time they’ll arrive is less certain.
SPWC will have more clear information once that solar energy reaches NASA’s Advanced Composition Explorer spacecraft, a satellite about 1 million miles from Earth.
Yet “even though that sounds like it’s far away . . . it doesn’t necessarily give us a ton of lead time,” Bettwy said. Given that solar wind is moving faster than the speed of sound, it will reach Earth only 20 to 45 minutes after passing that 1-million-mile marker in space.
Scientists have previously warned that the strongest geomagnetic storms could wreak havoc on our power and communication systems. That is not what’s expected in this case.
Those of us here on Earth may experience power outages and minor internet or GPS glitches, Bettwy said.
“Geomagnetic storms can impact infrastructure in near-Earth orbit and on Earth’s surface, potentially disrupting communications, the electric power grid, navigation, radio and satellite operations,” NOAA said in a statement. “SWPC has notified the operators of these systems so they can take protective action.”
Watches for milder geomagnetic storms are fairly common. In March, a geomagnetic storm briefly reached G4 strength for a few hours.
That event was the third geomagnetic storm to reach G4 status during the current 11-year solar cycle, which began in 2019, according to the SWPC. At that time, the agency said the event posed no risk of adverse impacts to the public.
“What’s unique about what is potentially about to hit us is that it’s a much more significant event, in terms of what’s going to be hitting the atmosphere,” Bettwy said.
Science
Do zinc products really help shorten a cold? It's hard to say

You feel a cold coming on, or maybe it’s already upon you: the telltale cough, sore throat and stuffy head. You swing by the drugstore, where a shelf full of over-the-counter products containing the mineral zinc claim to be able to shorten the duration of your symptoms.
The promise of relief is tempting. But is it one these products can make good on?
A new analysis of studies published on zinc and cold viruses concludes that there isn’t enough evidence to say whether over-the-counter zinc treatments have any effect on preventing the common cold.
For those who pop lozenges or inhale nasal sprays once a cold has come on, the available research together indicates that the products may reduce the duration of symptoms by up to two days, said Daryl Nault, an assistant professor at Maryland University of Integrative Health and first author of the paper, published Wednesday by the nonprofit organization Cochrane.
But those studies are so inconsistent in terms of the dosage, type of zinc, patient population and definition of cold symptoms that “confidence in the evidence is mostly low to very low,” the review states. “It is likely that additional studies are required before any firm conclusions can be drawn.”
In other words: Nearly 30 years after zinc lozenges first hit the market, we still can’t say for sure if these things do what they say they do.
“We aren’t saying [zinc] does” have any effect on the common cold, Nault said. “We aren’t saying it doesn’t. We’re saying we need more consistent evidence that is replicable. That’s a cornerstone of good science.”
The age of zinc cold products dawned in 1996, when researchers from the Cleveland Clinic Foundation convinced 100 clinic employees to volunteer as research subjects within 24 hours of developing a cold.
Half were given placebos, and half were given lozenges containing 13.3 milligrams of zinc from zinc gluconate every two waking hours as long as their symptoms persisted. Those receiving the zinc got better after 4.4 days on average, while the placebo group felt sick for an average of 7.6 days.
Most people consume a sufficient amount of zinc, a vital nutrient, through a regular diet. The mineral is plentiful in red meat and poultry, and present in many grains and fruits. (Oysters contain more zinc per serving than any other known food, with a single serving containing nearly 300% of the daily recommended intake.)
Scientists aren’t exactly sure how the mineral works to alleviate cold symptoms. But the idea of an over-the-counter way to shorten the misery of a common cold has proved wildly popular.
Total U.S. sales of zinc products, such as Zicam and Cold-Eeze, were $340 million in 2023, said Hannah Esper, managing editor of the trade publication Nutrition Business Journal. Demand for zinc and other supplements exploded during the COVID-19 pandemic, with sales for zinc growing 168.3% during 2020.
Based in the U.K., Cochrane uses rigorous research methods to evaluate existing scientific evidence and produce reports to help people make decisions about their health, according to its website.
For this review, the Cochrane team looked at 34 studies conducted across 13 countries that examined zinc products and the treatment or prevention of the common cold.
Drawing strong conclusions from the available research is difficult, as the studies tend to measure different things, said author Susan Wieland, an assistant professor at the University of Maryland School of Medicine and director of the Cochrane Complementary Medicine Field.
The cold “is a very common condition that is a difficult one to study,” Wieland said. It comes and goes quickly, making it difficult to enroll research subjects. Dosages and the type of zinc administered to study subjects varied widely.
“The designs of each study are different. So different dosages, different dosage forms, different patient populations, different criteria of exclusion and inclusion, different outcomes [and] definitions of cold,” said Dr. Jason Yee, an antimicrobial stewardship pharmacist at Cedars Sinai Medical Center in Los Angeles who was not involved with the review. “It’s really hard to draw the same conclusion based on different studies.”
Physicians said they weren’t surprised by the findings.
“I agree with the study. … It is consistent with my clinical experience in the hospital,” said Dr. Samia Faiz, an internal medicine specialist at UC Riverside Health. “In general, healthy people may be able to take zinc supplements if they make them feel better or if they get some comfort. They should not take these supplements if they have distaste or stomach upset.”
While over-the-counter zinc products are generally harmless to patients battling colds, said Dr. Pritish Tosh, an infectious disease physician and researcher at the Mayo Clinic, popping lozenges “shouldn’t come at the expense of doing things that really matter, which is getting plenty of rest, plenty of fluids and taking care of yourself.”
So why do we continue to fork over our cash for these things when we don’t really have more than a hunch that they work?
When a cold hits, “it’s natural for consumers to just reach for anything that may help alleviate those symptoms. But average consumers aren’t really educated on the literature and studies that are out there showing that there’s limited evidence and efficacy with these products,” Yee said.
Buying the lozenges or huffing the nasal spray can make us feel like we have more agency in a situation where we’re at the mercy of time and our immune systems, Nault said.
“Having a sense of control makes a lot of people feel better, and feel like they’re doing something,” Nault said. “Even if they aren’t.”
Times researcher Scott Wilson contributed to this report.
Science
Health officials warn Californians of risks of fake Botox. Here's what to look for
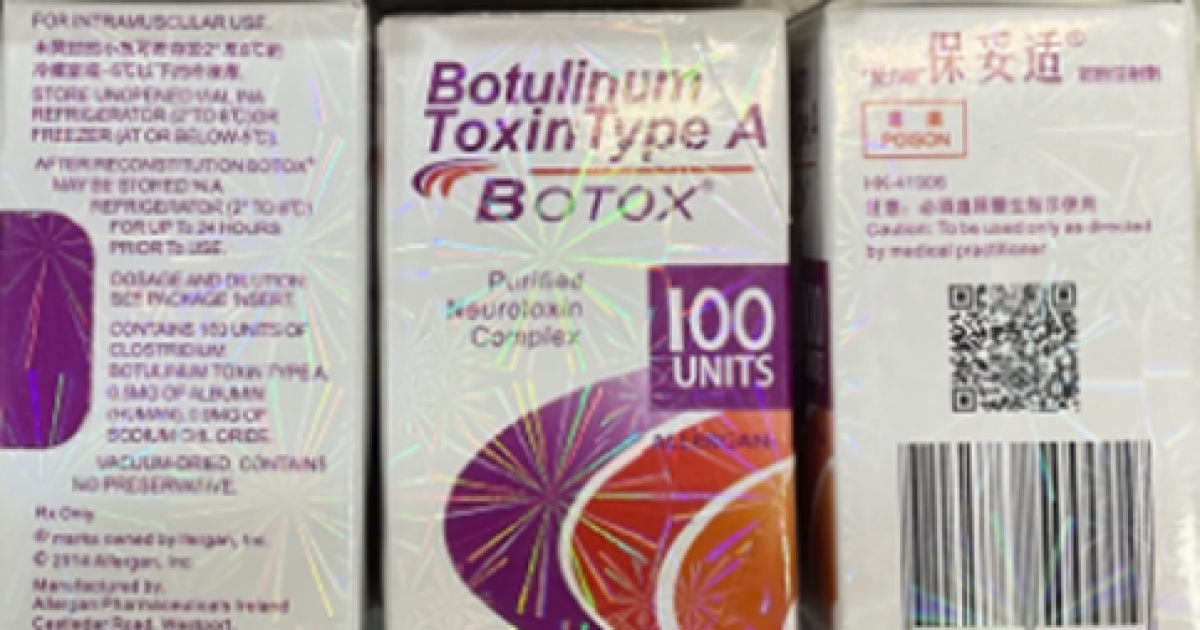
Fake versions of Botox have popped up in California, raising alarm among public health officials who warn that counterfeit versions of the injections can lead to symptoms such as slurred speech and breathing problems.
“Counterfeit or incorrectly administered Botox, even in small amounts, can result in serious health problems and even death,” Dr. Tomás J. Aragón, director of the California Department of Public Health, warned in a statement Wednesday.
Botox, or botulinum toxin, is used cosmetically to temporarily smooth fine lines on the face. It has also been employed to treat medical conditions such as muscle spasms. The product is derived from a toxin produced by bacteria.
Last month, the Centers for Disease Control and Prevention said that 22 people from 11 states had reported harmful reactions such as weakness and blurry vision after getting injections, landing some of them in the hospital. They had gotten their injections from unlicensed or untrained people or outside of healthcare settings, such as in a home or spa, according to the federal agency.
So far, there is no indication that such problems were linked to the genuine Botox product approved by the federal Food and Drug Administration, health officials said. Instead, regulators have found that some patients received counterfeit Botox products or ones from unverified sources. Investigations are underway.
“We’re not even sure what it really is,” but it’s not Botox, said Dr. Adam Friedman, chair of dermatology at George Washington School of Medicine and Health Sciences.
And “when you have an injectable agent that is not what it claims to be, has no quality assurance, no oversight … there could be a whole bunch of different things that come along for the ride,” including bacteria or allergens.
Because the health effects could be delayed, “I don’t think we’ve actually scratched the surface yet” of possible consequences from injecting an unknown substance into the body, Friedman said.
The California Department of Public Health said that since a multistate investigation launched in November, it had received two reports of harmful reactions to counterfeit or mishandled botulinum toxin, which were included in the total figure reported nationally by the CDC.
Under California law, Botox can be injected only by a physician, or by a registered nurse or physician assistant working under the supervision of a doctor. But state law “does not restrict where Botox treatments may be performed,” according to the Medical Board of California. In a statement, Aragón urged people to get Botox injections only from “licensed and trained professionals in healthcare settings.”
Public health officials also advised consumers to check with healthcare providers that they were getting Botox from “an authorized source” and to ask if they were licensed and trained to administer the injections.
“If in doubt, do not get the injection,” the public health department urged.
Aragón also stressed that Botox should never be purchased online or through “unlicensed individuals.” Dr. Debra Johnson, former president of the American Society of Plastic Surgeons, said that online sellers abroad have been creating “pirated Botox,” putting it in similar packaging, and then selling it to anyone who will pay.
Physicians have been getting emails and faxes saying, “‘We’ve got Botox for cheaper, we’ve got filler for cheaper’ — and it’s all these unregulated places that don’t have any FDA oversight,” Johnson said. Responsible doctors know that’s illegal, she said, but “I’m sure there’s some people who would hop at the chance.”
Botox is manufactured by AbbVie Inc. The California Department of Public Health said that outer cartons of the genuine product include product descriptions for either “BOTOX® COSMETIC / onabotulinumtoxinA / for Injection” or “OnabotulinumtoxinA / BOTOX® / for injection” and list the manufacturer as either “Allergan Aesthetics / An AbbVie Company” or “abbvie.” They also list the active ingredient as “OnabotulinumtoxinA.”
Fake products might show the active ingredient as “Botulinum Toxin Type A,” include languages other than English, or indicate 150-unit doses, according to the California Department of Public Health. (AbbVie manufactures real Botox products in 50-, 100- and 200-unit dose forms, federal officials said.) Another tipoff to a fake product is the lot number “C3709C3” on packaging or vials, regulators have advised.
Thankfully, “there’s some really key, distinct features on this fake Botox that distinguish it from the real thing, which has not been contaminated,” Friedman said. If a consumer is concerned, “there’s nothing wrong with saying, ‘Hey, can I check out the box?’”
In general, if “something seems to be too good to be true” or “it seems like a bargain when it comes to your health, those should be signals to run,” he said.
Anyone suffering symptoms from counterfeit Botox — which are similar to the effects of botulism poisoning from improperly canned foods — should contact a medical professional or go immediately to an emergency room, CDPH said. Symptoms can include drooping eyelids, trouble swallowing, fatigue, weakness and difficulty breathing.
Fake Botox products can be reported to the FDA through its website or by calling (800) 551-3989. In California, people can also tip off the California Department of Public Health by submitting a consumer complaint.
-

 News1 week ago
News1 week agoPolice enter UCLA anti-war encampment; Arizona repeals Civil War-era abortion ban
-

 Politics1 week ago
Politics1 week agoThe White House has a new curator. Donna Hayashi Smith is the first Asian American to hold the post
-

 News1 week ago
News1 week agoSome Florida boaters seen on video dumping trash into ocean have been identified, officials say
-

 Education1 week ago
Education1 week agoVideo: President Biden Addresses Campus Protests
-
)
) Movie Reviews1 week ago
Movie Reviews1 week agoThe Idea of You Movie Review: Anne Hathaway’s honest performance makes the film stand out in a not so formulaic rom-com
-

 World1 week ago
World1 week agoUN, EU, US urge Georgia to halt ‘foreign agents’ bill as protests grow
-

 World1 week ago
World1 week agoIn the upcoming European elections, peace and security matter the most
-
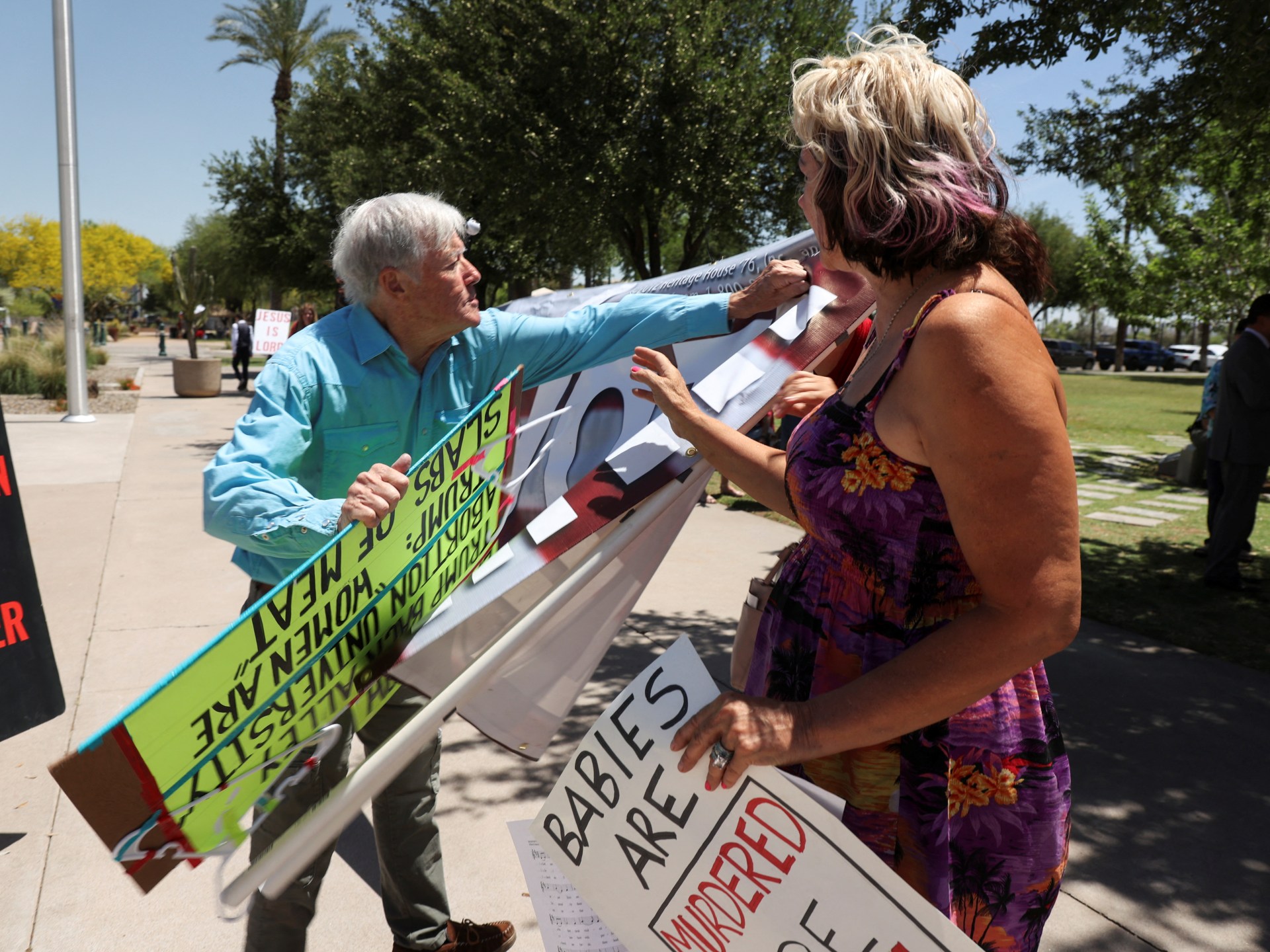
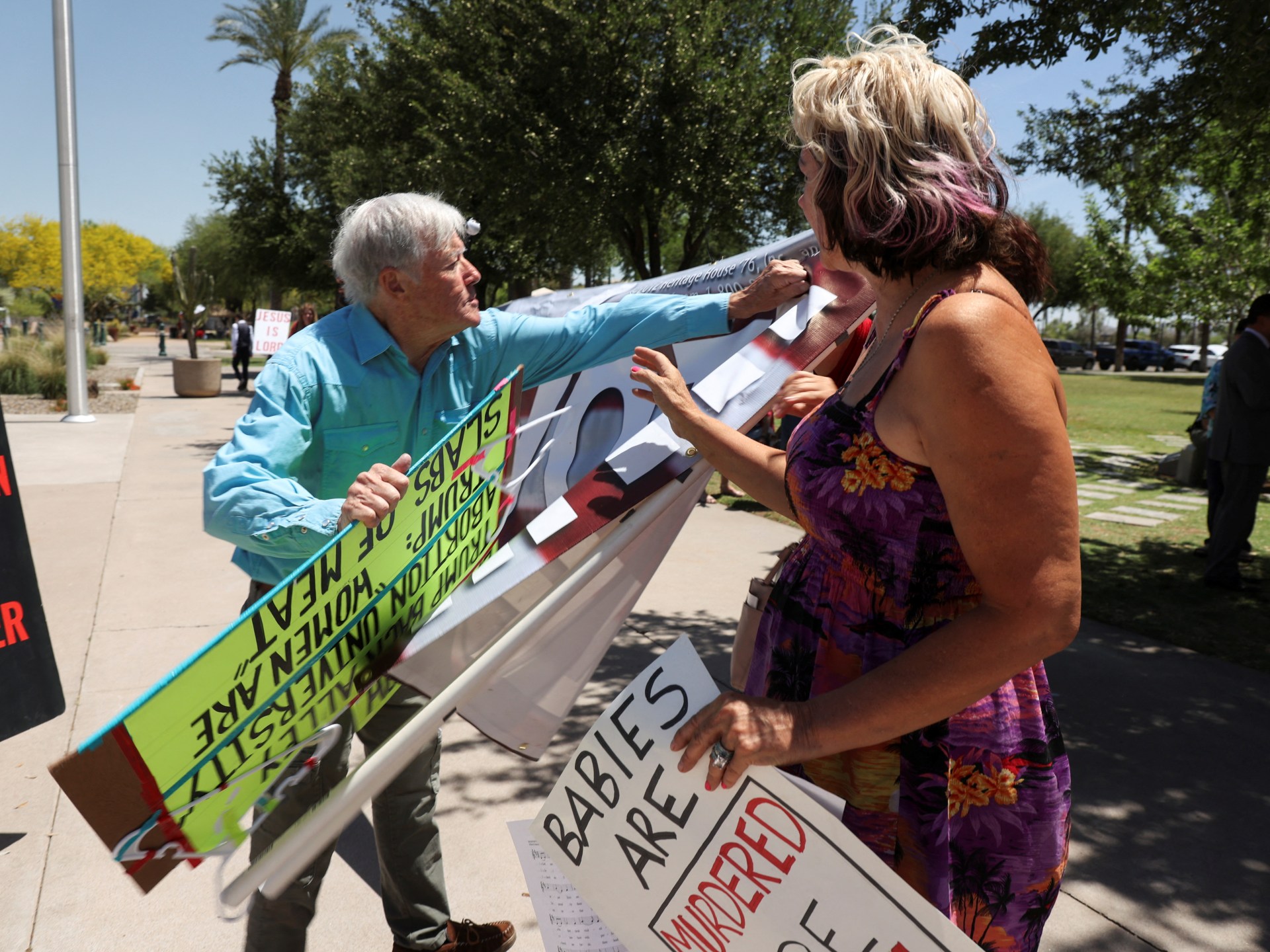 World1 week ago
World1 week agoArizona Senate repeals near-total 1864 abortion ban in divisive vote
/cdn.vox-cdn.com/uploads/chorus_asset/file/25443517/Ghost_of_Tsushima_Director_s_Cut_Wallpaper.jpg)













