Philadelphia, Pa
4th dose of COVID vaccine will be needed, Pfizer’s CEO says; not clear when, if FDA will authorize
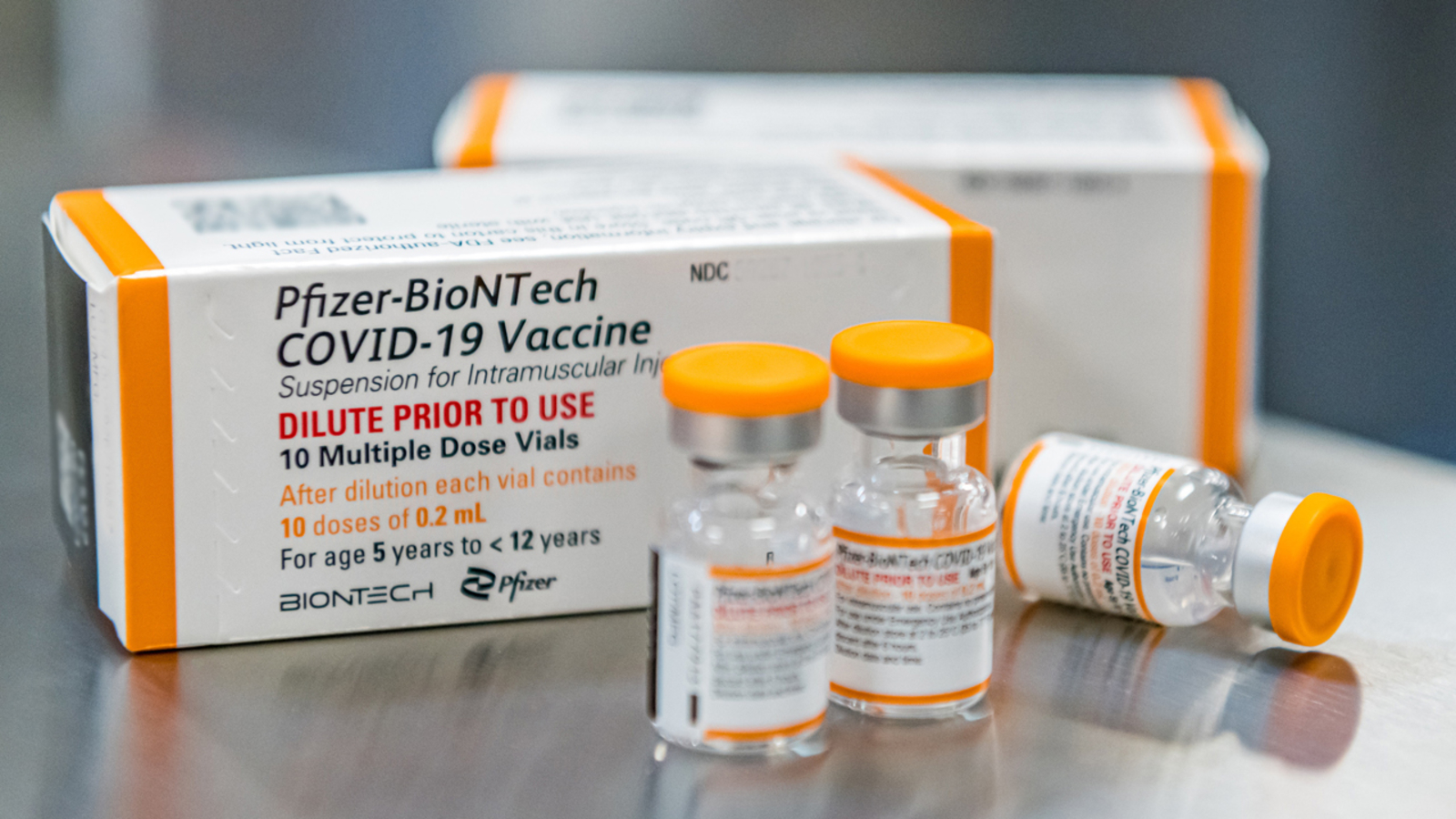
“Many variants are coming, and Omicron was the primary one which was capable of evade — in a skillful means — the immune safety that we’re giving,” Bourla instructed CBS’ “Face the Nation.”
“The safety we’re getting from the third (dose) it’s ok — truly fairly good for hospitalizations and deaths,” Bourla stated.
However safety after three doses is “not that good towards infections” and “does not final very lengthy” when confronted with a variant like Omicron.
“It’s needed, a fourth (dose) for proper now,” Bourla instructed CBS.
Presently, anybody ages 12 and up who bought a second dose of the Pfizer vaccine no less than 5 months in the past can get a 3rd dose.
Anybody ages 18 and up who bought the two-dose Moderna vaccine ought to get a booster shot six months after the second dose, in accordance with the US Facilities for Illness Management and Prevention.
And anybody who bought the single-dose Johnson & Johnson vaccine ought to get a booster shot after two months, the CDC stated.
Some reasonably or severely immunocompromised individuals who have had three doses of the Pfizer/BioNTech or Moderna Covid-19 vaccines can already get a fourth dose of vaccine, in accordance with the CDC.
However it’s not clear if or when the US Meals and Drug Administration may authorize a fourth dose of Covid-19 vaccine for wholesome teenagers and adults.
“We’re simply submitting these knowledge to the FDA, after which we’ll see what the consultants additionally would say outdoors Pfizer,” Bourla stated.
Youngsters below 5 may quickly be capable of get vaccinated
Presently, kids ages 5 to 11 are eligible for 2 pediatric doses of Pfizer’s Covid-19 vaccine however will not be but eligible for a booster. Pfizer is testing a 3rd dose in that age group now.
And youngsters below age 5 will not be but eligible for a Covid-19 vaccine — although that would change this spring, Bourla stated.
Preliminary trial knowledge in kids ages 2 to five confirmed two doses of a smaller, child-sized vaccine did not give the anticipated immunity within the 2- to 5-year-olds — although it did for infants ages 6 months to 2 years.
So Pfizer determined so as to add a 3rd child-sized dose for youngsters below age 5 in its ongoing trial.
Pfizer ought to have knowledge on its three-dose vaccine trial for youngsters ages 6 months to five years by April, Bourla stated.
If approved by the FDA and advisable by the CDC, Covid-19 vaccines for youngsters ages 6 months to five years may begin as early as Might, Bourla stated.
A shot at a longer-lasting vaccine towards all variants
Pfizer and Moderna have stated they’re engaged on a vaccine that will particularly defend towards the Omicron variant. It is not clear but if one is required.
Bourla stated Pfizer can be hoping to make a vaccine that may defend towards Omicron and all different variants of SARS-CoV-2 — the virus that causes Covid-19.
The purpose is to create “one thing that may defend for no less than a yr,” Bourla instructed CBS on Sunday.
“And if we’re capable of obtain that, then I believe it is rather straightforward to comply with and keep in mind in order that we will return to essentially the way in which (we) used to reside,” he stated.
The-CNN-Wire
& 2022 Cable Information Community, Inc., a WarnerMedia Firm. All rights reserved.

Philadelphia, Pa
FDA authorizes first COVID-19 vaccines for preschoolers, infants; CDC review is next

The Meals and Drug Administration’s motion follows its advisory panel’s unanimous suggestion for the pictures from Moderna and Pfizer. Which means U.S. children below 5 — roughly 18 million kids — are eligible for the pictures, about 1 1/2 years after the vaccines first grew to become out there within the U.S. for adults, who’ve been hit the toughest in the course of the pandemic.
The FDA additionally licensed Moderna’s vaccine for school-aged youngsters and youths. Pfizer’s pictures had beforehand been the one ones out there for these ages.
There’s one step left: The Facilities for Illness Management and Prevention recommends how you can use vaccines and its vaccine advisers are set to debate the pictures for the youngest children Friday and vote on Saturday. A ultimate signoff would come from CDC Director Dr. Rochelle Walensky.
At a Senate listening to Thursday, Walensky stated her employees was working over the Juneteenth federal vacation weekend “as a result of we perceive the urgency of this for American dad and mom.”
She stated pediatric deaths from COVID-19 have been larger than what is mostly seen from the flu annually.
“So I really assume we have to shield younger youngsters, in addition to shield everybody with the vaccine and particularly shield elders,” she stated.
For weeks, the Biden administration has been getting ready to roll out the vaccines. States, tribes, group well being facilities and pharmacies preordered tens of millions of doses. FDA’s emergency use authorization permits producers to start transport vaccine throughout the nation. Vaccinations may start as early as Monday or Tuesday.
Some dad and mom have been anxiously awaiting the prospect to guard their little ones.
Whereas younger youngsters usually do not get as sick from COVID-19 as older children and adults, their hospitalizations surged in the course of the omicron wave and FDA’s advisers decided that advantages from vaccination outweighed the minimal dangers. Research from Moderna and Pfizer confirmed unintended effects, together with fever and fatigue, have been largely minor.
MORE: FDA committee clears the best way for youths 6-17 to get one other COVID vaccine choice in Moderna
The 2 manufacturers use the identical know-how however there are variations.
Pfizer’s vaccine for youths youthful than 5 is one-tenth of the grownup dose. Three pictures are wanted: the primary two given three weeks aside and the final no less than two months later.
Moderna’s is 2 pictures, every 1 / 4 of its grownup dose, given about 4 weeks aside for youths below 6.
The vaccines are for kids as younger as 6 months. Moderna subsequent plans to check its pictures for infants as younger as 3-months-old. Pfizer has not finalized plans for pictures in youthful infants. A dozen nations, together with China, already vaccinate children below 5.
Dr. Beth Ebel, professor of pediatrics at College of Washington in Seattle, stated the tot-sized vaccines can be particularly welcomed by U.S. dad and mom with youngsters in daycare the place outbreaks can sideline dad and mom from jobs, including to monetary pressure.
“Lots of people are going to be glad and quite a lot of grandparents are going to be glad, too, as a result of we have missed these infants who grew up while you weren’t capable of see them,” Ebel stated.
___
AP Medical Writers Laura Ungar and Carla Okay. Johnson contributed.
___
The Related Press Well being and Science Division receives assist from the Howard Hughes Medical Institute’s Division of Science Training. The AP is solely liable for all content material.
Copyright © 2022 by The Related Press. All Rights Reserved.
Philadelphia, Pa
Amazon Prime announces 2022 Prime Day dates

Last year’s income spectacular, which returned to be able to its usual summer set, was the biggest two-day sales period for thirdparty sellers in the carrier’s history.
Online spending over the event surpassed $11 thousand, a 6.1% raise compared to Prime Moment 2020, which was presented in October, as outlined by Flag Analytics.
The event commences at 3 am OU on July 12 plus continues for 48 a long time spanning several countries, which includes for the first time frame Poland and Sweden.
Discounts are offered on electronic devices, toys, home goods plus clothing among other things.
“With the small corporations and national brands all of our members love and have confidence in, we’re excited to present a few of our best Leading Day deals yet to be able to even more customers all around the world,” Jamil Ghani, vice president connected with Amazon Prime, said throughout a press release.
Amazon holds the event every year to construct loyalty having its Prime subscribers plus hook new shoppers straight into the program.
Prime Moment, which has taken position since 2015, accounts with regard to roughly 1% to 2% of Amazon’s annual income, analysts say.
Sales throughout the Prime Day celebration expand beyond Amazon likewise. Rivals including Walmart, Concentrate on, Best Buy and Macy’s also hold sales connected with their own to utilize on the surge throughout online traffic driven by simply Prime Day shoppers.
The-CNN-Wire
& 2022 Cable News Networking, Inc., a WarnerMedia Organization. All rights reserved.
Philadelphia, Pa
Revlon files for bankruptcy protection amid heavy debt load

The corporate has been a mainstay on retailer cabinets since its founding 90 years in the past in New York Metropolis because it oversaw a steady of family names, from Almay to Elizabeth Arden.
Revlon did not hold tempo with altering tastes, nevertheless, gradual to comply with ladies as they traded flashy purple lipstick for extra muted tones within the Nineties.
Along with shedding market share to massive rivals like Procter & Gamble, newcomer beauty traces from Kylie Jenner and different celebrities efficiently capitalized on the large social media following of the well-known faces that fronted the merchandise.
Already weighed down by rising debt, Revlon’s issues solely intensified with the pandemic as lipstick gave option to a brand new period in style, this one that includes medical-grade masks.
Gross sales dropped 21% in 2020, the primary 12 months of the pandemic, although these gross sales rebounded 9.2% in its most up-to-date reporting 12 months with vaccines widespread. Within the newest quarter that led to March, gross sales rose practically 8%, however nonetheless lag pre-pandemic ranges in extra of $2.4 billion a 12 months.
The worldwide provide chain disruptions which are hobbling a whole bunch of worldwide corporations in current months had been an excessive amount of for Revlon, which barely escaped chapter in late 2020 by persuading bondholders to increase its maturing debt.
There could also be extra company restructurings within the client merchandise sector forward with the specter of an financial recession and the rising prices of borrowing cash.
Revlon mentioned Thursday that upon courtroom approval, it expects to obtain $575 million in financing from its present lenders, which is able to enable it to maintain its day-to-day operations operating.
“Right now’s submitting will enable Revlon to supply our customers the long-lasting merchandise now we have delivered for many years, whereas offering a clearer path for our future progress,” mentioned Debra Perelman, who was named Revlon president and CEO in 2018.
Her father, billionaire Ron Perelman, backs the corporate by means of MacAndrews & Forbes, which acquired the enterprise by means of a hostile takeover within the late Nineteen Eighties. Revlon went public in 1996.
Perelman mentioned that demand for its merchandise stay robust, however its “difficult capital construction” provided restricted capacity to navigate.
Throughout its heyday within the twentieth century, Revlon trailed solely Avon in gross sales. It now holds the twenty second spot amongst cosmetics makers, in keeping with a current rating by style commerce journal WWD.
Revlon grew to become the primary magnificence firm to characteristic a Black mannequin in 1970, Naomi Sims. Within the Nineteen Eighties, the corporate energized the cosmetics business by placing each well-known and yet-to-be-discovered fashions like Iman, Claudia Schiffer, Cindy Crawford and Christy Turlington entrance and middle, promising to make all ladies “unforgettable.”
Perelman, in an interview with The Related Press late final 12 months earlier than international provide chains locked up, mentioned she was optimistic in regards to the future. The corporate doubled-down in the course of the pandemic to get extra on-line with companies like one-on-one digital consultations by means of its Elizabeth Arden line, she mentioned.
Perelman additionally mentioned that the corporate was studying from superstar launches to be extra nimble and that Revlon had regained market share.
None of Revlon’s worldwide working subsidiaries are included within the proceedings, apart from Canada and the UK. The submitting was made within the U.S. Chapter Court docket for the Southern District of New York,
The corporate listed property and liabilities between $1 billion and $10 billion, in keeping with its chapter submitting.
Copyright © 2022 by The Related Press. All Rights Reserved.
-
/cdn.vox-cdn.com/uploads/chorus_asset/file/24924653/236780_Google_AntiTrust_Trial_Custom_Art_CVirginia__0003_1.png)
/cdn.vox-cdn.com/uploads/chorus_asset/file/24924653/236780_Google_AntiTrust_Trial_Custom_Art_CVirginia__0003_1.png) Technology5 days ago
Technology5 days agoGoogle’s counteroffer to the government trying to break it up is unbundling Android apps
-

 News6 days ago
News6 days agoNovo Nordisk shares tumble as weight-loss drug trial data disappoints
-

 Politics6 days ago
Politics6 days agoIllegal immigrant sexually abused child in the U.S. after being removed from the country five times
-

 Entertainment7 days ago
Entertainment7 days ago'It's a little holiday gift': Inside the Weeknd's free Santa Monica show for his biggest fans
-

 Lifestyle6 days ago
Lifestyle6 days agoThink you can't dance? Get up and try these tips in our comic. We dare you!
-

 Technology1 week ago
Technology1 week agoFox News AI Newsletter: OpenAI responds to Elon Musk's lawsuit
-
/cdn.vox-cdn.com/uploads/chorus_asset/file/25672934/Metaphor_Key_Art_Horizontal.png)
/cdn.vox-cdn.com/uploads/chorus_asset/file/25672934/Metaphor_Key_Art_Horizontal.png) Technology2 days ago
Technology2 days agoThere’s a reason Metaphor: ReFantanzio’s battle music sounds as cool as it does
-

 News3 days ago
News3 days agoFrance’s new premier selects Eric Lombard as finance minister



















