Health
Pregnant woman with brain cancer refuses abortion: ‘Killing my baby wouldn’t have saved me’

“We recommend that you get an abortion.”
That was the advice Tasha Kann received from doctors in Michigan shortly after she learned that she had brain cancer in 2022.
The young mother, who was 20 weeks pregnant with her second child, had just been diagnosed with anaplastic astrocytoma grade III, a rare and aggressive malignant tumor. Her doctors urged her to end her pregnancy so that she could receive chemotherapy and radiation.
“I told them absolutely not,” Kann shared with Fox News Digital in an interview.
CANCER AFTER 9/11: NEW JERSEY MAN BEATS MULTIPLE MYELOMA YEARS AFTER ATTACK, VOWS HE’LL ‘FIGHT TO LIVE’
Kann went on to give birth to a healthy baby girl.
More than a year after her diagnosis — defying the doctors’ predictions — she is still alive.
Tasha Kann is pictured with baby daughter Gracey, her husband Taylor and their 2-year-old son, Deklan. (Lainey Kann Photography)
The start of the battle
Kann’s cancer battle began in 2021, with what she thought was a migraine.
As she was lying in bed waiting for the headache to pass, she started to feel tingling in her arms and legs, and was suddenly unable to move or stand.
Assuming she was having a stroke, Kann yelled for help and managed to call 911.
At the hospital, a CT scan revealed a large brain mass.
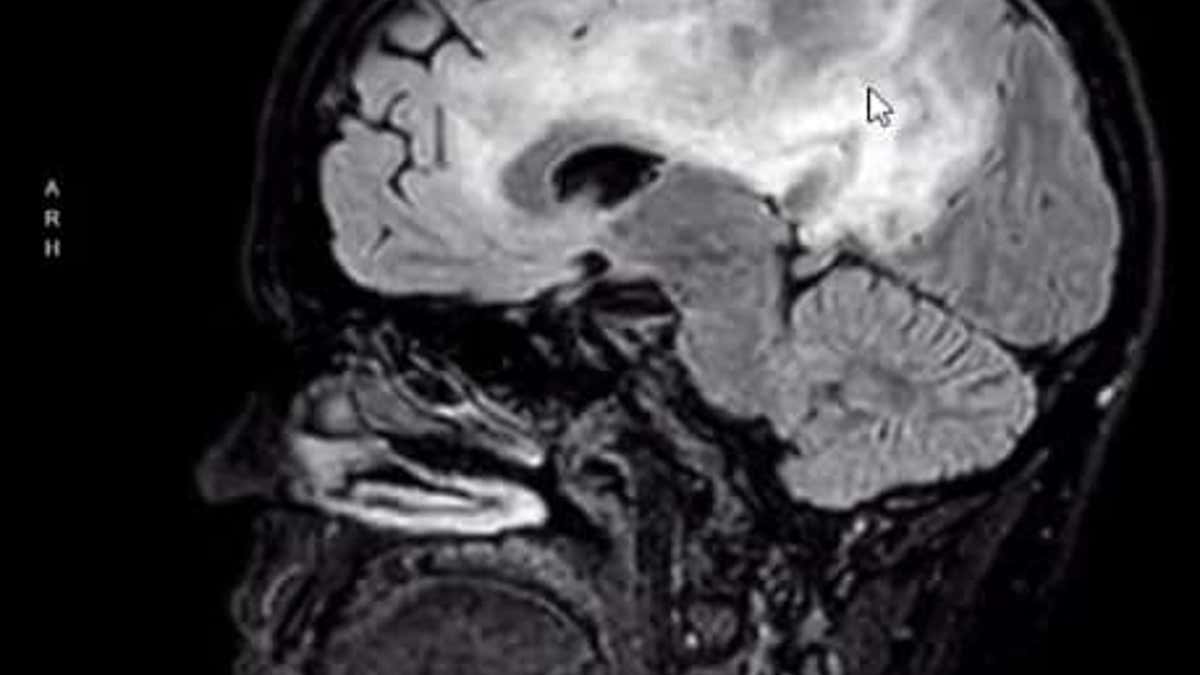
At 20 weeks pregnant, Kann was diagnosed with anaplastic astrocytoma grade III, a rare and aggressive malignant tumor. (Tasha Kann)
“I was a little scared, but I never lost hope,” Kann said of her diagnosis. “I knew I had to be strong for my baby.”
Kann said she still vividly recalls the moment that a group of three doctors entered her hospital room and stood at the foot of her bed.
“They all looked at me and told me my best chances of survival would be to get an abortion and start treatment immediately — which might give me five to eight years of survival,” she told Fox News Digital.

Tasha Kann’s healthy baby daughter was born in October 2022. (Tasha Kann)
When making the decision about her care, Kann said her faith was the biggest factor.
“Aborting my baby was never an option to me because it goes against God’s will,” she said.
“I had many deep conversations with Jesus that week in the hospital, and knew that if I held onto the Lord and his promises, he would keep my baby safe.”
Kann said she was determined to keep her baby alive and deliver her safely — after that, she would worry about saving herself.
“Aborting my baby was never an option to me because it goes against God’s will.”
Even as her scans remained stable for the remainder of her pregnancy, Kann said she was “disgusted” that the doctors continued to recommend an abortion.
“If the cancer was already as bad as they said, killing my baby wouldn’t have saved me anyway,” she noted.

After refusing to get an abortion, Kann went on to give birth to a healthy baby girl — and more than a year after her diagnosis, she is still alive. (Tasha Kann)
In her work as a hospice nurse, Kann said she saw firsthand the toll that chemotherapy and radiation took on many patients — and she pointed out that “it doesn’t always work.”
“I knew it would be a ‘no’ for me,” she said. “I decided to go home and do my own research and figure it out, while keeping my baby alive.”
Choosing life
After receiving her diagnosis, Kann immediately began researching holistic approaches to fighting her cancer, including making dietary changes, getting exercise and taking supplements.
She mainly sticks to a Keto-like diet, she said, and tries to incorporate light physical activity every day.
Kann’s second baby — a healthy girl named Gracey — was born in Oct. 2022, joining her 2-year-old son, Deklan.
MORE YOUNGER PEOPLE ARE RECEIVING CANCER DIAGNOSES, STUDY FINDS — ESPECIALLY THIS TYPE
At the time of her baby’s birth, according to the doctors’ predictions, Kann theoretically had around eight months left to live.
“Every single day, I look at my beautiful baby and think about how easy it was for them to tell me to abort — like she was nothing,” Kann told Fox News Digital.

Tasha Kann’s baby, Gracey, is pictured at 6 months old. “If I had listened — like most patients do, because they trust their doctors and don’t do their own research — my baby wouldn’t be here,” Kann said. (Tasha Kann)
“If I had listened — like most patients do, because they trust their doctors and don’t do their own research — my baby wouldn’t be here,” she said. “It’s a miracle from God that we are both here.”
She added, “I’m grateful my dad raised me to have enough confidence in myself and put all my trust into Jesus. That’s what I did and He delivered.”
Devastating setback
This past summer, the Kann family was dealt a crushing blow with the news that the cancer had spread.
It is now classified as Gliomatosis Cerebri, which is a highly aggressive tumor that affects the central nervous system and lobes of the brain.
Treatment options for this type of cancer are limited.
Kann has maintained her decision to not receive chemotherapy or radiation, instead seeking out alternative immunotherapy at the Burzynski Clinic in Houston, Texas.
“I will continue to follow and pray, give thanks and worship, as long as I’m living — especially when the doctors said I shouldn’t be.”
After visiting the clinic in person, Kann had a port installed in her chest so she can administer the immunotherapy treatments at home in Michigan.
Around the clock, she gets 12-minute infusions every four hours.
“As of now, we cannot find an oncologist in Michigan who will work with us and the Burzynski Clinic, so we will most likely have to go back to Texas every couple of weeks,” Kann said. “Meanwhile, we are continuing scans in Michigan.”
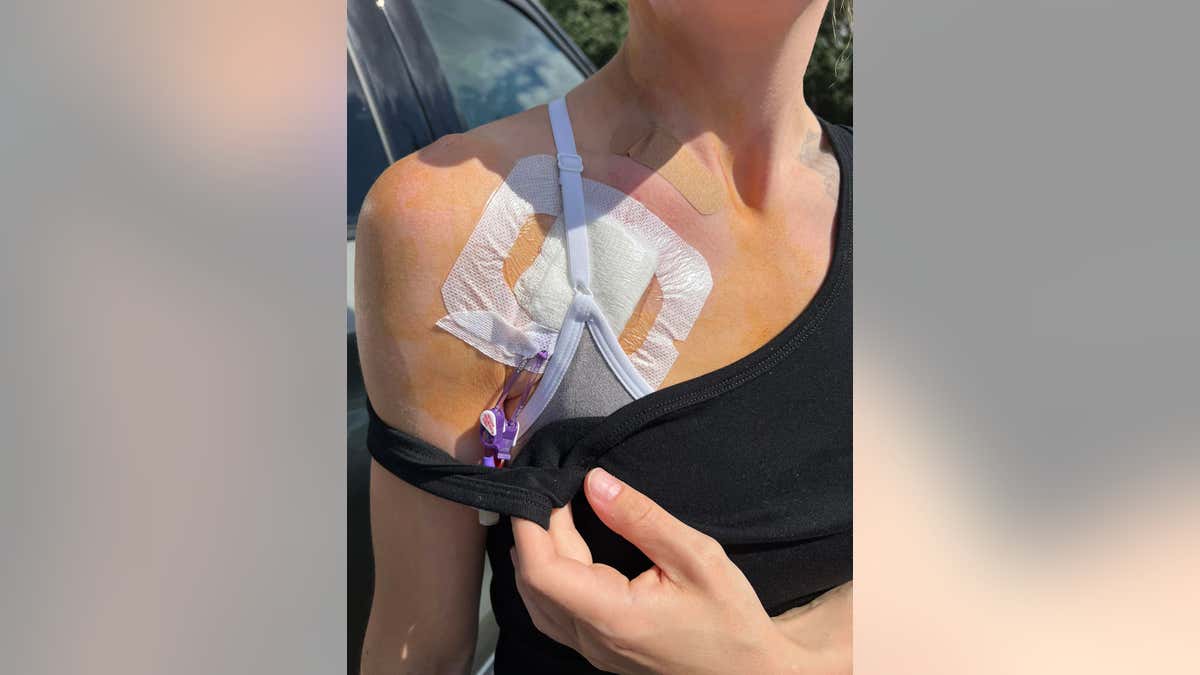
Kann had a port installed in her chest so she can administer the immunotherapy treatments at home in Michigan. Around the clock, she gets 12-minute infusions every four hours. (Tasha Kann)
The immunotherapy treatments are $17,000 per month. For the recommended 12 months of treatment, the total cost will exceed $200,000.
Because it’s considered experimental therapy that is not FDA-approved, insurance does not cover any of the expense.
“Our community has been a huge help, putting on fundraisers to help raise money for care,” Kann said.
Her family members have also set up a Go Fund Me, which has so far raised more than $92,000.
ASK AN EXPERT: ‘SHOULD I EXERCISE DURING MY CANCER TREATMENT?’
The Police Officer’s Association of Michigan has also called for donations, as Kann’s husband has served in law enforcement for a decade — both as a deputy and as a state trooper.
Dr. Marc Siegel, clinical professor of medicine at NYU Langone Medical Center and a Fox News medical contributor, noted that alternative therapies can be an “important consideration” for cancer that is inoperable or not responsive to standard treatments.

“My husband is my main support system,” Kann said. “He’s amazing, and I wouldn’t be able to heal like I am without him.” (Tasha Kann)
“Sometimes the latest treatments are not yet FDA-approved, and can and should be sought out under compassionate use with special approval,” he told Fox News Digital.
“On top of this, there are times when alternative approaches that are not on traditional medicine’s radar may be useful, but I am wary of using them as first options,” he added.
‘Walking by faith’
These days, Kann said she is “walking by faith,” focusing on raising her two young children.
“The doctors told me I had a prognosis of 12 months, but I beat that in June 2023,” Kann said. “Every time I talk to them, they make it seem like I’m going to die any day, but I’m still able to live a semi-normal life — walking, eating, talking — while having cancer in my central nervous system.”

“The only thing I ever wanted to be in life was a mom,” Kann told Fox News Digital. She’s pictured here holding her daughter Gracey. (Tasha Kann)
Although Kann said she feels “normal” a lot of the time, each day is different. Her main complaints are fatigue and weakness. She has had some small seizure activity, periodic vision issues and facial numbness.
“The oncologist said she doesn’t know how it’s possible I’m still alive,” Kann said. “How can there be any other answer than our Lord and Savior Jesus Christ?”
“I’m still able to live a semi-normal life — walking, eating, talking — while having cancer in my central nervous system.”
“I will continue to follow and pray, give thanks and worship, as long as I’m living — especially when the doctors said I shouldn’t be,” she went on.
“I’ll continue to prove them wrong.”
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
Beyond the physical sickness, Kann said cancer has been mentally draining for her.
“Sometimes people see me and because I’m not going through chemo and radiation, they think I’m fine,” she said. “But every day it’s a battle in my mind — I have to push myself and my body. It would be much easier to stay in bed and sleep, but that won’t help with healing the cancer.”
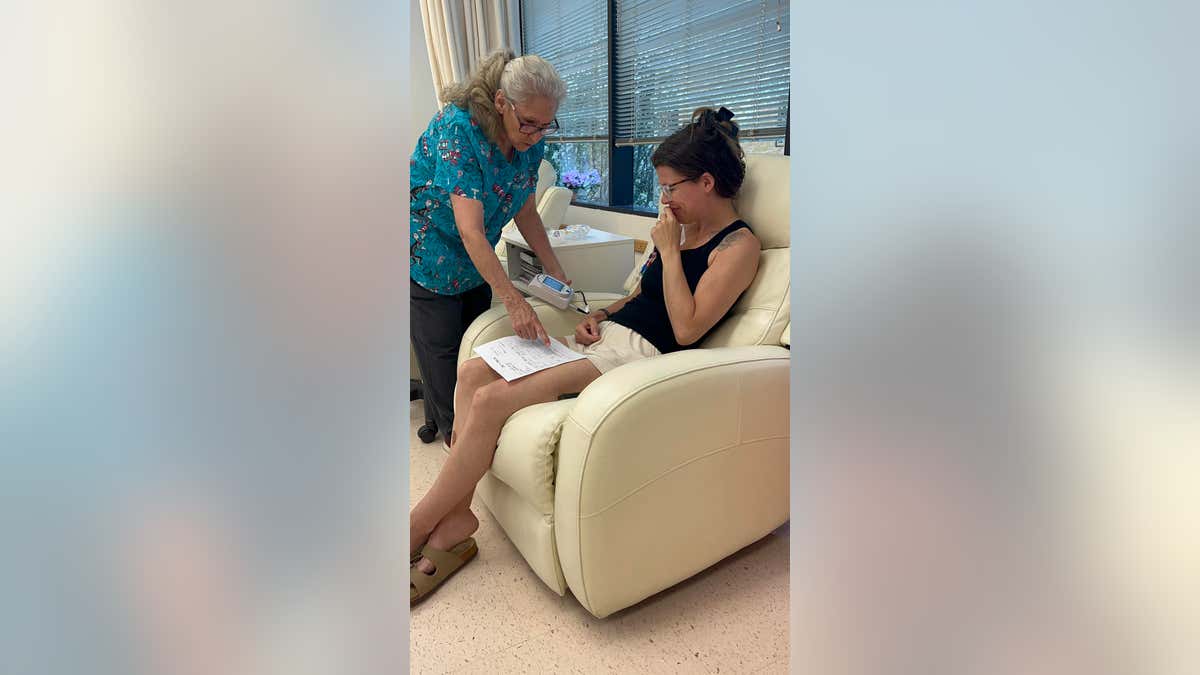
Kann is pictured receiving alternative immunotherapy at the Burzynski Clinic in Houston, Texas. (Tasha Kann)
Kann credits her husband and children for giving her a daily reason to fight.
“My husband is my main support system,” she said. “He’s amazing, and I wouldn’t be able to heal like I am without him. And the smiles and laughter of my kids help keep me strong and remind me to keep going.”
Kann said her hope for the future is that she will become cancer-free and be able to raise her “two beautiful babies.”
She added, “The only thing I ever wanted to be in life was a mom.”
Fox News Digital reached out to the Burzynski Clinic for additional comment.

Health
FDA bans red food dye due to potential cancer risk

FDA looks to ban red food dye
Celebrity fitness trainer Jillian Michaels joins ‘Hannity’ to discuss the possibility of the FDA banning red food dye.
The U.S. Food and Drug Administration (FDA) has officially banned red dye — called Red 3, or Erythrosine — from foods, dietary supplements and ingested medicines, as reported by the Associated Press on Wednesday.
Food manufacturers must remove the dye from their products by January 2027, while drug manufacturers will have until January 2028 to do so, AP stated.
Any foods imported into the U.S. from other countries will also be subject to the new regulation.
RED FOOD DYE COULD SOON BE BANNED AS FDA REVIEWS PETITION
“The FDA is taking action that will remove the authorization for the use of FD&C Red No. 3 in food and ingested drugs,” said Jim Jones, the FDA’s deputy commissioner for human foods, in a statement.
The U.S. Food and Drug Administration has officially banned red dye — called Red 3, or Erythrosine — from foods, dietary supplements and ingested medicines (iStock)
“Evidence shows cancer in laboratory male rats exposed to high levels of FD&C Red No.3,” he continued. “Importantly, the way that FD&C Red No. 3 causes cancer in male rats does not occur in humans.”
The synthetic dye, which is made from petroleum, is used as a color additive in food and ingested drugs to give them a “bright cherry-red color,” according to an online statement from the FDA.

Food manufacturers must remove the dye from their products by January 2027, while drug manufacturers will have until January 2028 to do so. (iStock)
The petition to ban the dye cited the Delaney Clause, which states that the agency cannot classify a color additive as safe if it has been found to induce cancer in humans or animals.
The dye was removed from cosmetics nearly 35 years ago due to potential cancer risk.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
“This is a welcome, but long overdue, action from the FDA: removing the unsustainable double standard in which Red 3 was banned from lipstick but permitted in candy,” said Dr. Peter Lurie, director of the group Center for Science in the Public Interest, which led the petition effort, as reported by AP.

Nearly 3,000 foods are shown to contain Red No. 3, according to Food Scores, a database of foods compiled by the Environmental Working Group. (iStock)
Dr. Marc Siegel, clinical professor of medicine at NYU Langone Health and Fox News senior medical analyst, applauded the FDA’s ban.
“It was a long time coming,” he told Fox News Digital. “It’s been more than 30 years since it was banned from cosmetics in the U.S. due to evidence that it is carcinogenic in high doses in lab rats. There needs to be a consistency between what we put on our skin and what we put into our mouths.”
“There needs to be a consistency between what we put on our skin and what we put into our mouths.”
Siegel said he believes the FDA’s decision could be tied to the incoming new head of the Department of Health and Human Services, Robert F. Kennedy Jr.
“They knew it would have happened anyway under RFK Jr.,” he said. “It is already banned or severely restricted in Australia, Japan and the European Union.”

The food additive also “drew kids in” to a diet of empty calories and ultraprocessed foods, one doctor stated. (iStock)
The food additive also “drew kids in” to a diet of empty calories and ultraprocessed foods, Siegel added.
“It has also been linked to behavioral issues in children, including ADHD.”
Nearly 3,000 foods are shown to contain Red No. 3, according to Food Scores, a database of foods compiled by the Environmental Working Group.
For more Health articles, visit www.foxnews.com/health
The National Confectioners Association provided the below statement to Fox News Digital.
“Food safety is the number one priority for U.S. confectionery companies, and we will continue to follow and comply with FDA’s guidance and safety standards.”
The petition to remove Red No. 3 from foods, supplements and medications was presented in 2022 by the Center for Science in the Public Interest and 23 other organizations and scientists.
Health
How Yvette Nicole Brown Lost Weight and Got Her Diabetes Under Control

Sign Up
Create a free account to access exclusive content, play games, solve puzzles, test your pop-culture knowledge and receive special offers.
Already have an account? Login
Use left and right arrow keys to navigate between menu items.
Use escape to exit the menu.
Health
As bird flu spreads, CDC recommends faster 'subtyping' to catch more cases

As cases of H5N1, also known as avian flu or bird flu, continue to surface across the U.S., safety precautions are ramping up.
The U.S. Centers for Disease Control and Prevention (CDC) announced on Thursday its recommendation to test hospitalized influenza A patients more quickly and thoroughly to distinguish between seasonal flu and bird flu.
The accelerated “subtyping” of flu A in hospitalized patients is in response to “sporadic human infections” of avian flu, the CDC wrote in a press release.
ONE STATE LEADS COUNTRY IN HUMAN BIRD FLU WITH NEARLY 40 CONFIRMED CASES
“CDC is recommending a shortened timeline for subtyping all influenza A specimens among hospitalized patients and increasing efforts at clinical laboratories to identify non-seasonal influenza,” the agency wrote.
The CDC now recommends accelerated subtyping of influenza A in response to “sporadic human infections” in the U.S. (iStock)
“Clinicians and laboratorians are reminded to test for influenza in patients with suspected influenza and, going forward, to now expedite the subtyping of influenza A-positive specimens from hospitalized patients, particularly those in an intensive care unit (ICU).”
LOUISIANA REPORTS FIRST BIRD FLU-RELATED HUMAN DEATH IN US
The goal is to prevent delays in identifying bird flu infections and promote better patient care, “timely infection control” and case investigation, the agency stated.
These delays are more likely to occur during the flu season due to high patient volumes, according to the CDC.
For more Health articles, visit www.foxnews.com/health
Health care systems are expected to use tests that identify seasonal influenza A as a subtype – so if a test comes back positive for influenza A but negative for seasonal influenza, that is an indicator that the detected virus might be novel.
Identifying bird flu infections will support better patient care and infection control, the CDC says. (iStock)
“Subtyping is especially important in people who have a history of relevant exposure to wild or domestic animals [that are] infected or possibly infected with avian influenza A (H5N1) viruses,” the CDC wrote.
In an HHS media briefing on Thursday, the CDC confirmed that the public risk for avian flu is still low, but is being closely monitored.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
The agency spokesperson clarified that this accelerated testing is not due to bird flu cases being missed, as the CDC noted in its press release that those hospitalized with influenza A “probably have seasonal influenza.”
Niels Riedemann, MD, PhD, CEO and founder of InflaRx, a German biotechnology company, said that understanding these subtypes is an “important step” in better preparing for “any potential outbreak of concerning variants.”

The CDC recommends avoiding direct contact with wild birds or other animals that may be infected. (iStock)
“It will also be important to foster research and development of therapeutics, including those addressing the patient’s inflammatory immune response to these types of viruses – as this has been shown to cause organ injury and death during the COVID pandemic,” he told Fox News Digital.
Since 2022, there have been 67 total human cases of bird flu, according to the CDC, with 66 of those occurring in 2024.
The CDC recommends that people avoid direct contact with wild birds or other animals that are suspected to be infected. Those who work closely with animals should also wear the proper personal protective equipment (PPE).
-
/cdn.vox-cdn.com/uploads/chorus_asset/file/25822586/STK169_ZUCKERBERG_MAGA_STKS491_CVIRGINIA_A.jpg)
/cdn.vox-cdn.com/uploads/chorus_asset/file/25822586/STK169_ZUCKERBERG_MAGA_STKS491_CVIRGINIA_A.jpg) Technology1 week ago
Technology1 week agoMeta is highlighting a splintering global approach to online speech
-

 Science6 days ago
Science6 days agoMetro will offer free rides in L.A. through Sunday due to fires
-
/cdn.vox-cdn.com/uploads/chorus_asset/file/25821992/videoframe_720397.png)
/cdn.vox-cdn.com/uploads/chorus_asset/file/25821992/videoframe_720397.png) Technology1 week ago
Technology1 week agoLas Vegas police release ChatGPT logs from the suspect in the Cybertruck explosion
-

 News1 week ago
News1 week agoPhotos: Pacific Palisades Wildfire Engulfs Homes in an L.A. Neighborhood
-

 Education1 week ago
Education1 week agoFour Fraternity Members Charged After a Pledge Is Set on Fire
-

 Politics1 week ago
Politics1 week agoTrump trolls Canada again, shares map with country as part of US: 'Oh Canada!'
-
/cdn.vox-cdn.com/uploads/chorus_asset/file/23935558/acastro_STK103__01.jpg)
/cdn.vox-cdn.com/uploads/chorus_asset/file/23935558/acastro_STK103__01.jpg) Technology6 days ago
Technology6 days agoAmazon Prime will shut down its clothing try-on program
-

 News1 week ago
News1 week agoMapping the Damage From the Palisades Fire













