Science
A Climate Warrior’s Journey From Summit Talks to Street Protests
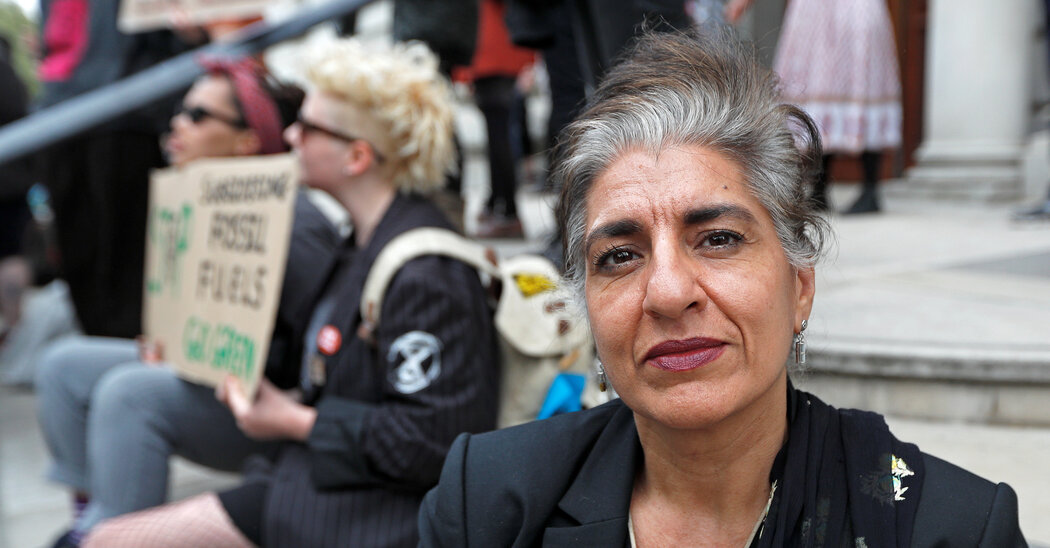
“I felt that the entire multilateral world, the worldwide framework for human rights, was simply collapsing round me,” Ms. Yamin mentioned.
When, from a gathering room at a United Nations local weather convention in Marrakesh, Morocco, Ms. Yamin watched Mr. Trump win the election, she was despondent. She felt that her 30-year profession as a authorities lawyer and local weather negotiator had amounted to nothing.
“All of it was going up in smoke,” she mentioned. “I couldn’t inform my shoppers, I couldn’t mislead the Marshall Islands, that we’d repair this.” Ms. Yamin took a 12 months off, spending most of her time in nature remedy lessons and tenting within the wilderness for weeks at a time.
Throughout her day without work, Ms. Yamin started studying about different social actions, just like the anti-apartheid marketing campaign and the suffragist motion, that used social mobilization and nonviolent resistance to advance their causes. “I felt that the local weather motion was nearly distinctive and fragile, relying totally on insider techniques and never on motion constructing,” she mentioned. “It wasn’t counting on the total units of instruments.”
It was this concept that reignited Ms. Yamin’s ardour for local weather and helped her get again to work. As an alternative of returning to local weather diplomacy, Ms. Yamin joined the nascent Extinction Rise up motion, a decentralized group that makes use of nonviolent motion and civil disobedience, in 2018.
Initially, Ms. Yamin grew to become the chief of Extinction Rise up’s political crew, utilizing her data of the diplomatic terrain to assist the motion be extra strategic in its activism and get extra funding. Even in her new activist function, although, Ms. Yamin felt she was relying too closely on her mental abilities as an alternative of placing her physique on the road. When an Intergovernmental Panel on Local weather Change report was issued in October 2018, Ms. Yamin was studying the report as activists crammed Parliament Sq. in London. As she noticed footage of younger individuals refusing to maneuver and ready to be arrested, she thought, “I need to be with them.”
Ice shelf collapses. For the primary time since satellites started observing Antarctica almost half a century in the past, an ice shelf has collapsed on the japanese a part of the continent. The 450-square-mile ice shelf was positioned in an space often known as Wilkes Land; the loss occurred in mid-March.
Perceive the Newest Information on Local weather Change
Ms. Yamin spent the next two years working with Extinction Rise up, organizing and protesting alongside different activists. She stepped down from her function with the group in 2020 due to disagreements with different leaders. Ms. Yamin mentioned she believed the motion was not targeted sufficient on local weather justice.

Science
New drug's potentially fatal side effects obscured by 'soothing acronym,' doctors say
Seventy-nine-year-old Genevieve Lane volunteered to take the Alzheimer’s drug Leqembi in a clinical trial because she was forgetting words and misplacing her keys.
Infusions of the drug gave her headaches so severe they sent her to bed. A week after the third dose, she was at a restaurant with her best friend when her speech slurred and she had a seizure. Five days later she was dead.
An autopsy found that Lane died of a mysterious side effect that has a name that sounds like it might be part of an Italian opera, but has doctors on edge.
The complication called ARIA has nothing to do with music. It is a term adopted by an influential group of pharmaceutical executives and academic scientists to describe potentially fatal bleeding and swelling in the brain caused by drugs like Leqembi.
“Mom believed the drug would help slow progression of her memory problems or do nothing,” said Lane’s daughter, Yvonne Battaglia. “She didn’t know it might kill her.”
Genevieve Lane, 79, of The Villages, Fla., not long before she died in September, 2022. An autopsy blamed Leqembi, a drug for Alzheimer’s disease.
(Lane family)
Lane’s death, and that of two other trial participants, has raised concerns among some doctors, who question whether Leqembi’s risks are worth its benefits, particularly for the population of older adults it was approved for.
Some of these doctors are urging that a new name be given to the drug’s potential side effects to better alert healthcare professionals to its risks.
ARIA is short for “amyloid-related imaging abnormalities,” with imaging referring to the MRI scans needed to find the brain bleeding and swelling.
“Clearly, it is more than just an imaging abnormality,” said Dr. Matthew Schrag, a Vanderbilt University neurologist, who helped with an autopsy that concluded Lane died of brain swelling and bleeding that was likely caused by Leqembi.
“My feeling is that ARIA is too euphemistic of a term. It conveys that this isn’t serious, and it certainly can be,” he said.
Leqembi, known generically as lecanemab, is a monoclonal antibody that works to remove a protein called amyloid from the brain. It received full Food and Drug Administration approval in July.
Eisai, a Japanese drugmaker that has partnered with Biogen, is promoting Leqembi to doctors and people concerned about their memories.
“Get ahead & stay ahead for longer,” an Eisai website says about the drug. It also says that “ARIA usually occurs early in treatment and is usually asymptomatic, although serious and life-threatening events rarely can occur.”
In a recent article, a Stanford neurologist and his colleagues detailed their concerns about ARIA, which they called a “soothing acronym” for brain bleeding and swelling.
“It does certainly have the ring of something that a pharmaceutical company or public relations person would come up with,” Dr. Michael Greicius said in an interview.
Leqembi is approved for mild dementia and also a diagnosis known as mild cognitive impairment, where patients have more memory problems than others their age but can compensate and continue their daily activities. People with MCI have been found to be at greater risk for developing dementia; but in many cases, their memory problems stay the same or even improve.
The FDA has required that the company warn doctors about ARIA. The agency says the condition, which affected more than 20% of those taking the drug in a large trial, can be managed by requiring patients to get repeated MRI scans to look for bleeding and swelling.
“The FDA maintains that the benefits of Leqembi outweigh its risks when used according to the approved labeling,” said Dr. Teresa Buracchio, director of the FDA’s neuroscience office.
Libby Holman, an Eisai spokesperson, called ARIA “globally established nomenclature.”
Because of the risk of ARIA, some Los Angeles medical centers are taking extra precautions.
At Keck Medicine of USC, a neurologist is available 24/7 to take calls from families of those taking Leqembi, since a headache or sudden confusion can be a sign of ARIA, said Dr. Helena Chui, chair of the neurology department.
At UCLA and Cedars-Sinai Medical Center, warnings pop up in a patient’s electronic health record to ensure that all medical staff know the patient is taking Leqembi, because it can interact with certain other medications to make brain bleeding far worse.
And at all three medical centers it takes more than a single doctor to prescribe the drug. Each patient’s case must be reviewed by a panel of doctors and other staff — similar to how complex cancer cases are evaluated.
“We want to keep safety first,” said Dr. Keith Vossel, UCLA professor of neurology. “This is the most complicated, complex drug that we’ve prescribed in the dementia field.”
Other physicians say they won’t prescribe the drug.
“If there was a medication that worked, I would be the first person to use it,” said Dr. Clifford Sigel, a neurologist in Santa Monica. “But I won’t be using this in my practice.”
He pointed to a large clinical trial of Leqembi that led to its approval. It found that patients who took the drug saw their memory decline 27% more slowly — or less than half a point on an 18-point cognitive scale — than their counterparts who took a placebo. Sigel and other doctors doubt patients or their families would notice the difference.
Eisai’s Holman disputed claims that the drug does not work. She noted that a panel of outside experts convened by the FDA had voted unanimously that trial data confirmed its clinical benefit.
The name ARIA traces to July 2010 when “turmoil ensued” at an international scientific conference that the Alzheimer’s Assn. holds each year, according to an article by two scientists working in the field.
The FDA had proposed that companies testing new anti-amyloid drugs exclude any volunteer from clinical trials who had more than two brain microbleeds, according to an Alzheimer’s Assn. report. The tiny hemorrhages are sometimes found in healthy people and those with Alzheimer’s or other illnesses.
The agency also said it would require any volunteer who experienced a brain microbleed during the clinical trial to cease taking the drug.
The companies and academics working on the trials viewed the new FDA requirements as “excessively restrictive,” said the report by the association, a nonprofit that has become a powerful force in dementia science.
The industry and academic scientists feared the FDA proposal would stall research on the experimental drugs, the report said, and limit their use.
The drug companies asked the association to debate the FDA’s guidance at its Research Roundtable. Pharmaceutical and medical testing companies can become members of the roundtable by paying the association a $50,000 annual fee.
The association said that “one key question” taken up by the roundtable was whether ARIA was a temporary symptom of the new drug — much the way nausea and hair loss are side effects of chemotherapy — or evidence that anti-amyloid medicines may have more serious adverse effects. That question was never settled.
“Current knowledge doesn’t provide definitive answers to this critical question,” the association said in the 2011 report explaining the roundtable’s work.
Despite the unknowns, the roundtable proposed that volunteers be allowed into the trials even if they had as many as four brain microbleeds. The group said volunteers could keep getting the drug infusions if they developed brain bleeding as long as they did not have significant worsening of symptoms such as headaches and confusion.
The roundtable also proposed calling the brain bleeding and swelling ARIA.
The FDA “subsequently revised and updated the original advice…in a manner consistent” with the roundtable’s suggestions, wrote three of its members.
“Scientific evidence at the time led the workgroup to propose excluding people who have four or more microbleeds from clinical trials,” the association told The Times in a statement. “The FDA agreed.”
The association declined to answer questions on whether the name ARIA should be changed.
An FDA official told The Times that the industry group’s advice was just one of the factors the agency considered before it revised its 2010 guidelines.
More than a decade later, little more is known about why ARIA occurs or how to recognize it.
One problem is that a patient with ARIA can look like they’re having a stroke. And when stroke patients are taken to an emergency room, the first treatment doctors often consider is a clot-dissolving medicine called tPA, which can make brain bleeding worse.
That’s what happened to a 65-year-old woman taking Leqembi in a trial who arrived at a Chicago ER with stroke-like symptoms, according to a report published in February 2023. Doctors gave her tPA.
“As soon as they put it in her, it was like her body was on fire,” the woman’s husband said in a news story in the journal Science. “She was screaming, and it took like eight people to hold her down.”
The woman died, and an autopsy showed extensive bleeding in her brain, leading doctors to conclude the combination of the two drugs may have caused her death.
Knowing about that risk, Southern California doctors have been teaching emergency room staff to find out if patients thought to be suffering a stroke may be taking Leqembi.
“We’ve had to train and discuss this with the ER, the neuroradiology team and urgent care,” said Dr. Sarah Kremen, who leads Cedars Sinai’s Alzheimer’s clinical trial program. “You must ask this person, ‘Are you taking this medication?’”
An FDA database that collects reports of adverse drug reactions from doctors and others shows 23 deaths of patients taking Leqembi.
Holman at Eisai said it would be incorrect to assume the deaths were caused by Leqembi. She noted that Alzheimer’s patients have a higher risk of death because of the natural course of the disease.
In the large trial, less than 1% of patients died — the same rate whether they were taking the drug or the placebo.
Buracchio at the FDA said the agency takes “all adverse event reports seriously.” But she said the agency’s evaluation of the reports “must take the treated population into account,” which in this case is typically older or elderly adults.
To teach doctors about ARIA, Eisai created a website called understandingaria.com. It tells doctors that ARIA “usually resolves without intervention or treatment modification.”
In a brochure for healthcare providers, Eisai assures physicians that infusions may continue if an MRI turns up evidence of microbleeds as long as there are four or fewer and that the discomfort doesn’t disrupt the patient’s activities.
For Genevieve Lane, an MRI discovered four brain microbleeds before she started taking Leqembi in the trial.
After Lane died, an autopsy found more than 30 microbleeds in her brain, including some that could not be seen on the MRI, according to a report in Nature Communications.
The report’s authors, who included Schrag at Vanderbilt, questioned whether the pre-treatment limit of four brain microbleeds was stringent enough and called for higher standards.
The FDA told The Times that the agency had reviewed the available data and had not identified a specific number of preexisting microhemorrhages that would make it unsafe for patients to take anti-amyloid drugs like Leqembi.
“However, we will continue to monitor the accruing safety data,” the agency said.
Other doctors have questioned what happens to the memories of those who suffer ARIA, even if the bleeding and swelling appears to resolve.
Dr. Madhav Thambisetty, a senior researcher at the National Institute of Aging, said he was concerned by a report in a French medical journal about two women with mild dementia who experienced serious ARIA during a trial. One suffered severe seizures; 11 months later, her memory score dropped by nine points on a 30-point scale. The other patient developed a brain bleed described as “massive”; she lost a significant part of her vision, and her memory score declined by 12 points on the same scale.
An FDA scientist reviewing reports of patients who suffered high numbers of microbleeds in the clinical trial also noted the possible harm to their cognition in her January 2023 report on Leqembi.
One of the patients that Dr. Deniz Erten-Lyons pointed to was a 68-year-old man who had four microbleeds before starting the infusions. After treatment, he began to lose his vision and was hospitalized because of a seizure. An MRI found 96 microbleeds.
Thambisetty said he and Dr. Rob Howard of University College London wrote to Eisai last year to request information about what happened to the cognition of those who suffered ARIA in trials.
Eisai has not responded to their request, he said.
“I’m concerned about the lack of full and transparent reporting,” Thambisetty said. “It’s really important to know what happens to these patients.”
Holman said the company’s analysis of trial data showed that ARIA did not impact cognition.
“Eisai is transparent,” she said. The company follows guidelines for sharing clinical data established by PhRMA, the industry trade association, Holman said.
Greicius, the Stanford professor, also asked Eisai for trial data that would break down results for each volunteer to better understand ARIA and whether patients benefited as more amyloid was removed from their brains.
The response from Eisai, he said, was, “Thanks for your interest, but we can’t release the data.”
Science
Judge halts ban on syringe programs as El Dorado County legal battle continues
El Dorado County cannot enforce its ban on programs that hand out clean syringes as a legal battle continues between the county and the California Department of Public Health, a Superior Court judge has ruled.
Judge Gary S. Slossberg granted a preliminary injunction to prevent El Dorado County from enforcing an ordinance that makes it unlawful to operate syringe programs in its unincorporated areas.
The judge said he was not weighing in on the heated arguments for or against syringe programs, which provide sterile needles to people who use drugs, but whether the Department of Public Health had a “reasonable probability” of prevailing in its argument that the county ordinance clashes with state law.
Friday’s decision does not end the courtroom dispute over whether the ban passed by the El Dorado County Board of Supervisors was preempted by state law, as public health officials have argued, or opposing claims by county officials that the syringe program was improperly approved by the state. Slossberg said Friday that the preliminary injunction is meant to remain in place pending a later trial.
The Department of Public Health filed suit against El Dorado County and its county seat of Placerville this year contending that their bans on syringe programs defied the state health and safety code.
The state health department first authorized the nonprofit Sierra Harm Reduction Coalition to operate a syringe program in the county four years ago. State officials have long endorsed such programs as a proven way to prevent HIV and hepatitis C from running rampant as people share contaminated syringes.
California law gives the public health agency the power to approve syringe programs anywhere that deadly or disabling infections might spread through used needles, “notwithstanding any other law.”
Local bans on syringe programs have nonetheless sprung up across California as city and county officials argue that handing out free syringes does more harm than good. El Dorado County leaders passed their rule in December, which was followed in February by a similar ordinance in Placerville.
The lawsuit lodged by the California Department of Public Health drew objections from El Dorado County leaders: Earlier this year, Dist. Atty. Vern Pierson called it “madness” and argued that California officials were “seeking to impose the normalization of hardcore drug use.”
In a cross-complaint filed against the Department of Public Health, the county said that the syringe program approved by the state had caused “profound nuisance and public safety impacts,” including a “drastic increase in discarded needles,” and that overdoses had risen since it started.
The county said in a legal filing that since the ban went into effect, “there has been a reduction of syringe waste, decreased incidents of public nuisance, and a resulting reduction of the burdens on law enforcement.”
It also accused the public health department of failing to follow state requirements when it approved the syringe program.
The judge did not weigh in on the cross-complaint lodged by El Dorado County at the Friday hearing. In a court filing, California officials said studies show that syringe programs provide important resources for needle disposal and play a crucial role in preventing overdoses. They credited the Sierra Harm Reduction Coalition with handing out thousands of boxes of Narcan, a brand of naloxone, a medication that reverses the effects of an opioid overdose.
The Department of Public Health argued in a legal filing that stopping the syringe program would be likely to ramp up HIV and hepatitis C infections among people who use drugs, increasing state costs for their care; lead to more deaths from drug overdoses; and reduce access to options for syringe disposal, among other harmful effects.
Because of the bans, “our most vulnerable, stigmatized, and marginalized community members are actively being denied lifesaving interventions,” Sierra Harm Reduction Coalition interim executive director Shilo Jama said in a court filing.
Slossberg said that although he was preventing the El Dorado County ordinance from being enforced, the county might have other mechanisms to address nuisance issues that were not addressed by the decision.
Pierson, the district attorney, said in a statement Friday that “we will propose narrowing the ordinance” in response to comments made by the judge.
The California Department of Public Health said in a statement that it was “pleased with the court’s decision that upholds that state’s role in protecting the public’s health while this case proceeds.”
The Friday ruling applies only to the ordinance passed by El Dorado County. Attorney Mona Ebrahimi, who represents the city of Placerville, said a hearing involving the city had been postponed.
Science
This one thing may derail your shot at healthy aging, scientists say

Before you settle in to binge the new season of “The Bear” or watch Team USA go for the gold at the Paris Olympics, think twice about the amount of time you spend on the couch in front of the TV. Your future self may thank you.
A new study by Harvard researchers links the popular pastime of sitting and watching television to the likelihood of reaching one’s senior years in a state of good health: the more time spent doing the former, the lower the odds of achieving the latter.
The problem doesn’t seem to be with sitting in general. After controlling for a variety of risk factors such as diet quality and smoking history, the researchers found no relationship between time spent in a chair at work and the chances of aging well. Ditto for sitting in cars or at home doing something besides watching TV, such as reading, eating meals or paying bills.
Yet for every additional two hours spent in front of the boob tube, a person’s chance of meeting the researchers’ definition of healthy aging declined by 12%, according to their study published this week in JAMA Network Open.
That does not bode well for the United States, where 62% of adults between the ages of 20 and 64 say they watch TV for at least two hours a day, as do 84% of senior citizens.
The findings are based on data from more than 45,000 women who participated in the Nurses Heath Study. All of them were at least 50 years old and had no major chronic diseases back in 1992, when they answered a slew of questions about their health and what they did all day.
For instance, the nurses were asked how much time they spent standing or walking around at work or at home. They were asked about various types of exercise, including jogging, swimming laps, playing tennis and doing yoga. They were asked if they mowed their own lawns.
And they were asked how many hours they spent doing all kinds of sitting.
A couple watches a movie on TV at their home in Norwalk while sharing a bowl of popcorn.
(Francine Orr / Los Angeles Times)
You might not be surprised to learn that the most popular type of sitting was sitting while watching television. More than half of the women — 53% — said they watched between six and 20 hours of TV a week. (The median among this group was around 15.4 hours per week.) Another 15% of the women said they watched between 21 and 40 hours of TV each week, and 2% watched even more.
The nurses were tracked for 20 years or until they died, whichever came first. By the end of the study period, 41% of them were still free of 11 major health conditions, including cancer, diabetes, heart failure, chronic obstructive pulmonary disease and multiple sclerosis. In addition, 44% of the nurses were in good mental health, 52% had no memory impairments and 16% had no physical impairments.
Only 8.6% of the women met all four of those criteria, which was what it took to achieve healthy aging.
On the whole, the women who watched more TV tended to be older, were more likely to be smokers or drinkers, consumed more calories and had higher body mass index scores than women who watched less TV. The more devoted TV watchers were also more likely to have high blood pressure and high cholesterol.
Once the researchers accounted for these and a host of other differences, they found that the women who spent an hour or less each week sitting in front of the TV were the most likely to achieve healthy aging. Compared to them, women who watched TV for two to five hours per week were 9% less likely to be healthy agers; those who watched for six to 20 hours per week were 19% less likely; those who watched for 21 to 40 hours per week were 40% less likely; and those who watched for at least 41 hours a week were 45% less likely.
The researchers also found that replacing TV time with pretty much anything else — including sleep, for women who got no more than seven hours of shut eye per night — would increase their odds of healthy aging. The more vigorous the new activity, the bigger the boost.
Although the actual percentage of women who succeeded in healthy aging was low, the study authors estimated that another 61% of the women could have joined that rarefied group if they had done four things:
- Spent at least three hours per day engaged in light physical activity at work.
- Invested at least 30 minutes a day in moderate to vigorous physical activity.
- Kept their weight in the normal range instead of being overweight or obese.
- Limited their TV-watching time to less than three hours a day.
The study didn’t show that excess TV time caused any of the nurses to miss out on healthy aging, only that there was a significant inverse correlation between the two. Still, there’s good reason to suspect that their favorite sedentary behavior bore at least some of the responsibility.
Previous studies have linked prolonged sitting — especially while watching television — to a variety of health problems, including diseases like breast cancer, colorectal cancer, type 2 diabetes, cardiovascular disease and early death. (That particular study found that compared to sitting for less than three hours a day, sitting for at least twice that long was associated with a 17% increased risk of premature death for men and a 34% increased risk of premature death for women.)
But the researchers from Harvard’s T.H. Chan School of Public Health have taken things a step further, said Dr. I-Min Lee, an epidemiologist at Brigham & Women’s Hospital in Boston who studies how physical activity can prevent chronic diseases and extend life.
“This study expands what we know because it looked at ‘healthy aging,’” said Lee, who was not involved in the study. “‘Health’ is not just the absence of disease; it includes dimensions of physical and mental health, function and well-being.”
All of the study subjects were women, but the biological mechanisms are likely to apply to men as well, Lee said. Even so, it would be good to actually test this relationship in men, as well as in people from a wider range of racial and ethnic backgrounds, she said. (The group of women in the original Nurses Health Study was overwhelmingly white.)
The youngest of the Baby Boomers are now turning 60, and the proportion of the U.S. population that’s at least 65 is projected to increase from roughly 17% today to nearly 21% in 2050, according to the U.S. Census Bureau.
“Population aging is an important public health issue,” the study authors wrote, and strategies to promote healthy aging “are urgently needed.”
-

 Politics1 week ago
Politics1 week agoNewson, Dem leaders try to negotiate Prop 47 reform off California ballots, as GOP wants to let voters decide
-

 News1 week ago
News1 week agoWould President Biden’s asylum restrictions work? It’s a short-term fix, analysts say
-

 World1 week ago
World1 week agoDozens killed near Sudan’s capital as UN warns of soaring displacement
-

 News1 week ago
News1 week agoRead Justice Clarence Thomas’s Financial Disclosures for 2023
-

 World1 week ago
World1 week ago‘Bloody policies’: Bodies of 11 refugees and migrants recovered off Libya
-

 Politics1 week ago
Politics1 week agoGun group vows to 'defend' Trump's concealed carry license after conviction
-

 Politics1 week ago
Politics1 week agoShould Trump have confidence in his lawyers? Legal experts weigh in
-

 Politics6 days ago
Politics6 days agoGOP releases Jan. 6 clip of Pelosi saying 'I take responsibility' as she discussed National Guard absence















