Health
Ozempic-Wegovy pill may be on the way: Trial shows promising results for new weight loss tablet

Ozempic and Wegovy — both injectable forms of semaglutide — have become synonymous with weight loss in recent months and years. Now a new drug is on the horizon that would offer the same medication in an oral (pill) format.
Created by Novo Nordisk, the same company that makes Ozempic and Wegovy, the new drug just completed its Phase 3 clinical trials. The results were announced on Sunday at the American Diabetes Association (ADA) Scientific Session in Los Angeles, California.
The oral medication, which is indicated for type 2 diabetes or weight loss for patients with obesity, would provide an alternative to people who are not open to getting injections.
WEIGHT LOSS MEDICATIONS OZEMPIC AND WEGOVY: WHAT TO KNOW BEFORE YOU STOP TAKING THEM
“The opportunity to have a tablet that can offer the effectiveness of weight loss for people with diabetes is really exciting,” Dr. Robert Gabbay, Boston-based chief scientific and medical officer for the ADA, told Fox News Digital during a phone interview from the scientific session.
“It opens up this type of therapy to many more people that could potentially benefit.”
A new drug that will offer the same medication in an oral (pill) format that current injectables offer just completed its Phase 3 clinical trials. (iStock)
Ozempic is marketed for people with type 2 diabetes, while Wegovy is indicated for weight loss. Both medications are semaglutides, just with different dosages.
“Our focus has been primarily on people with diabetes, but it turns out that the vast majority of people with type 2 diabetes also have obesity,” Gabbay explained.
“So having a medication that can address a central issue for people with diabetes is really important — especially for those who are somewhat resistant to or concerned about doing an injection.”
DOCTORS URGE CAUTION ON DIABETES DRUGS FOR WEIGHT LOSS AFTER STUDY HIGHLIGHTS SIDE EFFECTS
The double-blind, randomized, Phase 3 trial followed 667 people who were randomly assigned to either 50 milligrams of the drug or a placebo, explained the lead study author, Dr. Filip Knop, professor at Gentofte Hospital at the University of Copenhagen.
The participants took the semaglutide once daily for a 68-week period and were monitored for both safety and effectiveness.
Ozempic is marketed for people with type 2 diabetes, while Wegovy is indicated for weight loss. Both medications, however, are semaglutides, just with different dosages. (Getty Images)
“It’s important to emphasize that the participants were people who had obesity but did not have type 2 diabetes,” Knop told Fox News Digital in a phone interview.
“They could have [had] pre-diabetes, but they were not allowed to be enrolled in the trial if they had type 2 diabetes.”
The average amount lost by the patients was 44 pounds.
For the 86% of participants who remained on the drug throughout the trial, they achieved an average body weight loss of 17.4% compared to just 1.8% for those on the placebo, explained Knop, who has been involved in diabetes and metabolic research since 1999.
The average amount lost by the patients was 44 pounds.
In addition to helping with weight loss, the semaglutide medication can also help to reduce cardiovascular disease in people with diabetes, Gabbay noted.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
“For those people who are at high risk or [who] already have cardiovascular disease, the parent drug has been shown to reduce mortality, and there’s no reason to expect that this wouldn’t be the case with the oral version,” he told Fox News Digital.
Common side effects during the trial were gastrointestinal in nature — such as nausea, vomiting, constipation and diarrhea — similar to what has been reported for Ozempic and Wegovy.

The new oral medication, which is indicated for type 2 diabetes or weight loss for patients with obesity, would provide an alternative to people who are not open to getting injections. Patients would take a daily pill. (iStock)
Some less common side effects reported during the first 20 weeks of the trial were short-term changes in skin sensation, such as hypersensitivity or paraesthesia (tingling or prickling), Knop said.
Following the successful results of the Phase 3 trial, the drugmakers will look to start the process of applying for FDA approval.
“We’re pleased to see that there are new options for people with diabetes and obesity,” Gabbay said.
“Having something that is orally available should increase the number of people who can benefit from these kinds of treatments.”

Health
Better Than Ozempic? Doctors Say These Medications Are Better for Weight Loss Than the Popular Semaglutide

Sign Up
Create a free account to access exclusive content, play games, solve puzzles, test your pop-culture knowledge and receive special offers.
Already have an account? Login
Forgot your password?
Get back to the Sign In
Use left and right arrow keys to navigate between menu items.
Use escape to exit the menu.
Health
Honeybees can detect lung cancer, researchers say
What happens when you pair honeybees and halitosis? Potentially a life-saving new method to screen for cancer, according to one study.
Researchers at Michigan State University have learned that honeybees can detect chemicals associated with lung cancer in human breath. The insects were able to sniff out human lung cancer biomarkers with a remarkable 82% success rate, according to a study published in the journal Biosensors and Bioelectronics.
“These results indicate that the honeybee olfactory system can be used as a sensitive biological gas sensor to detect human lung cancer,” the study authors wrote.
“Insects have an amazing sense of smell the same way dogs do,” said MSU professor Debajit Saha, according to an MSU news release.
YOUNG VAPER WHO REQUIRED DOUBLE LUNG TRANSPLANT SHARES WARNINGS AS E-CIGARETTE SALES RISE
A honeybee drinking nectar from a flower in Markham, Ontario, Canada. (Creative Touch Imaging Ltd./NurPhoto via Getty Images)
Saha, an assistant professor in the College of Engineering and MSU’s Institute for Quantitative Health Science and Engineering, sought to determine whether honeybees could distinguish chemicals in a healthy person’s breath from that of someone sick with lung cancer.
His team developed a “recipe” for a synthetic breath mixture that contained six compounds present in the breath of someone with cancer and a synthetic “healthy” breath mixture.
“It took a steady hand to create the recipe,” said Elyssa Cox, Saha’s former lab manager. “We tested the synthetic lung cancer versus healthy human breath mixtures on approximately 20 bees.”
The researchers placed each live bee in a custom 3D-printed harness and attached a tiny electrode to its brain to measure activity.
SOME BREAST CANCER PATIENTS COULD BE AT RISK OF ANOTHER TYPE OF CANCER, STUDY REVEALS
Lung cancer is the leading cause of cancer death worldwide. An estimated 235,580 people will be diagnosed with lung cancer in 2024 in the U.S., according to the Lung Cancer Research Foundation. (Mohammed Haneefa Nizamudeen/iStock)
“We pass those odors on to the antenna of the honeybees and recorded the neural signals from their brain,” said Saha. “We see a change in the honeybee’s neural firing response.”
The researchers found that the bees were able to detect the cancer-indicating compounds even in small amounts.
“The honeybees detected very small concentrations; it was a very strong result,” said Saha. “Bees can differentiate between minute changes in the chemical concentrations of the breath mixture, which is in the parts per 1 billion range.”
The bees also could tell the difference between the synthetic lung cancer breath and healthy breath.
UK TO INTRODUCE BILL TO PHASE OUT LEGAL SALE OF TOBACCO

A honey bee visits a blooming catmint plant growing in Santa Fe, New Mexico. (Robert Alexander/Getty Images)
Scientists hope this research will lead to the development of a sensor based on a honeybee brain that can be used to test human breath for the presence of lung cancer.
“What’s amazing is the honeybees’ ability to not only detect cancer cells, but also distinguish between cell lines of various types of lung cancer,” said Autumn McLane-Svoboda, a graduate student on Saha’s team. “The future implications for this are huge, as our sensor could allow for patients to receive specific cancer diagnoses quickly, which is imperative for correct treatment routes.”
Lung cancer is the leading cause of cancer death worldwide. An estimated 235,580 people will be diagnosed with lung cancer in 2024 in the U.S., according to the Lung Cancer Research Foundation.
Smoking is the leading risk factor for lung cancer and is responsible for 80% of lung cancer deaths.
Early detection of high-risk lung cancer can reduce the chance of death by up to 20%.
Health
COVID vaccine companies told to focus on KP.2 variant for fall shots, per FDA announcement

The U.S. Food and Drug Administration (FDA) has recommended that COVID vaccine manufacturers update their formulas for fall doses, in an attempt to target the KP.2 strain of the JN.1 variant.
The Thursday announcement came just a week after the agency’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted to recommend a “monovalent JN.1-lineage vaccine” at its June 5 meeting.
As of the end of March 2024, the KP.2 variant was responsible for just 4% of infections in the U.S., according to the COVID Data Tracker from the Centers for Disease Control and Prevention (CDC).
CDC WARNS OF ‘DUAL MUTANT’ FLU STRAIN THAT COULD EVADE ANTIVIRAL DRUGS: ‘NEED TO CLOSELY MONITOR’
Meanwhile, over 50% of infections at that time were attributed to its parental strain, JN.1.
Just a few weeks later, KP.2 is now the cause of around 28% of infections, while the JN.1 variants have largely dropped in prevalence, the tracker shows.
The U.S. Food and Drug Administration has recommended that COVID vaccine manufacturers update their formulas for fall doses, in an attempt to target the KP.2 strain of the JN.1 variant. (iStock)
Dr. Marc Siegel, clinical professor of medicine at NYU Langone Medical Center and a Fox News medical contributor, recently spoke with Dr. Peter Marks, director of the Center for Biologics Evaluation and Research (CBER) at the Food and Drug Administration, about the new vaccine formulations.
“It makes sense to target the KP.2 strain because it is becoming the predominant strain — it is surging in California and will spread across the country,” Siegel told Fox News Digital.

KP.2 is now the cause of around 28% of infections, while the JN.1 variants have largely dropped in prevalence, CDC Tracker data shows. (iStock)
The KP.2 strain is “highly immunoevasive,” the doctor warned — which means that immunity from previous variants and subvariants don’t offer much protection.
COVID-FLU COMBO VACCINE SHOWS ‘POSITIVE’ RESULTS IN PHASE 3 TRIALS, MODERNA SAYS: A ‘TWO-FOR’ OPTION
“On the other hand, the vaccine will cause a production of immune cells and antibodies that will continue to protect you against previous variants and subvariants,” Siegel added.
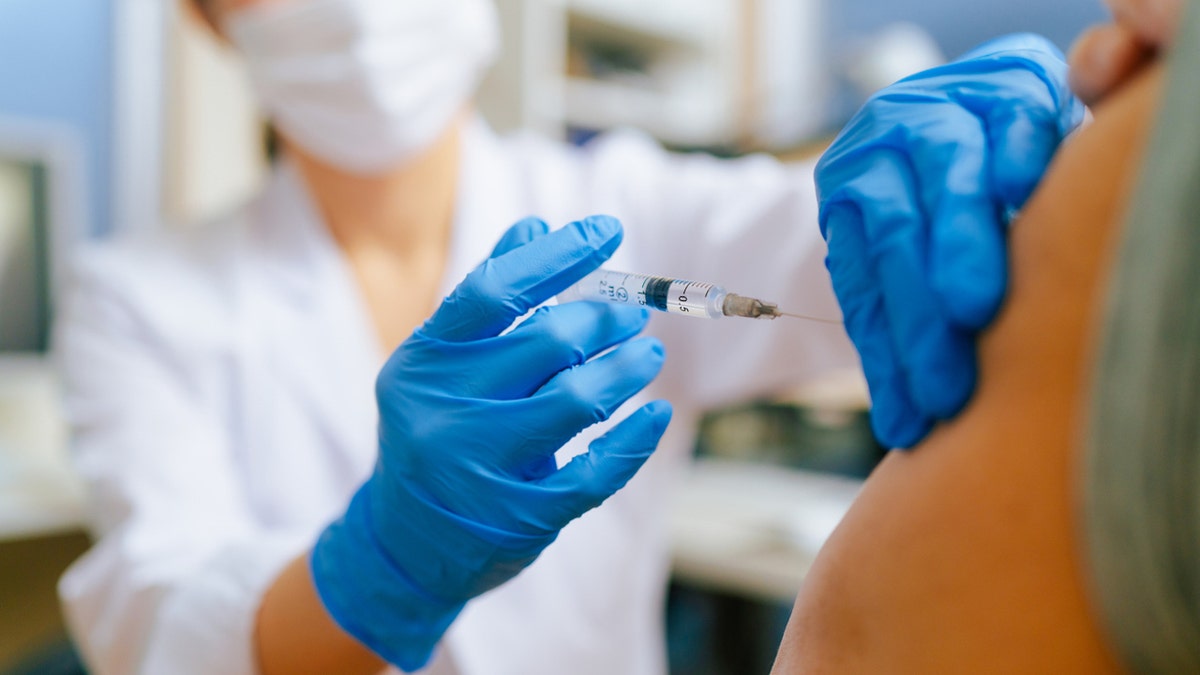
The updated vaccine is especially important for high-risk groups, those who have chronic illnesses, the elderly and anyone who comes in contact with them, according to doctors. (iStock)
It is especially important for high-risk groups, those who have chronic illnesses, the elderly and anyone who comes in contact with them, according to the doctor.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
In a statement to Fox News Digital, vaccine maker Novavax — which makes protein-based vaccines — said the company “just filed” its application for a JN.1 COVID vaccine.
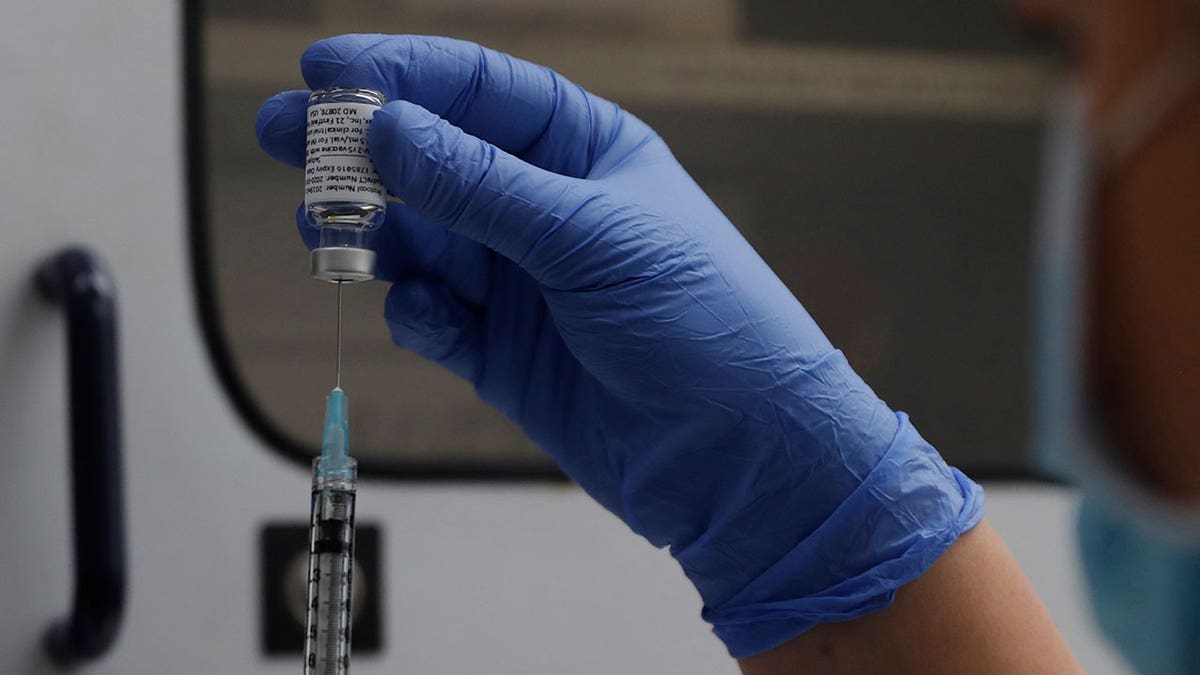
A vial of the Phase 3 Novavax coronavirus vaccine is seen ready for use in the trial at St. George’s University hospital in London, on Oct. 7, 2020. (AP Photo/Alastair Grant, File)
“Novavax’s updated JN.1 COVID-19 vaccine is active against current circulating strains, including KP.2 and KP.3,” the company said in a press release.
“The submission is in line with guidance from the U.S. FDA, European Medicines Agency (EMA) and the World Health Organization (WHO) to target the JN.1 lineage this fall.”
For more Health articles, visit www.foxnews/health.
Fox News Digital reached out to Pfizer and Moderna — both of which produce mRNA-based vaccines — requesting comment on their plans for fall formulations.
-

 News1 week ago
News1 week agoIsrael used a U.S.-made bomb in a deadly U.N. school strike in Gaza
-

 World1 week ago
World1 week agoFrance to provide Ukraine with its Mirage combat aircraft
-

 World1 week ago
World1 week agoWorld leaders, veterans mark D-Day’s 80th anniversary in France
-

 World1 week ago
World1 week agoRussia-Ukraine war: List of key events, day 833
-

 News1 week ago
News1 week agoNonprofit CFO Accused of 'Simply Astonishing' Fraud
-

 Movie Reviews1 week ago
Movie Reviews1 week agoInsane Like Me? – Review | Vampire Horror Movie | Heaven of Horror
-

 Politics1 week ago
Politics1 week agoGeorge Clooney called White House to complain about Biden’s criticism of ICC and defend wife’s work: report
-

 Politics1 week ago
Politics1 week agoNewson, Dem leaders try to negotiate Prop 47 reform off California ballots, as GOP wants to let voters decide