Health
How the Shortage of a $15 Cancer Drug Is Upending Treatment

Tony Shepard learned he had vocal cord cancer this spring, but he was encouraged when his doctor said he had an 88 percent chance at a cure with chemotherapy and radiation.
That outlook began to dim in recent weeks, though, after the oncology practice he goes to in Central California began to sporadically run out of the critical medication he needs.
Since Mr. Shepard’s doctor informed him of the shortage, each treatment session has felt like a game of “Russian roulette,” he said, knowing that failure would mean the removal of his vocal cords and the disappearing of his voice.
“I try not to even think about it,” said Mr. Shepard, 62, a manager of a gas station in Madera, a town in California’s Central Valley. “It’s something scary that you don’t really want to think about — but you know it’s a reality.”
The nation’s monthslong shortage of highly potent cancer drugs is grinding on, forcing patients and their doctors to face even grimmer realities than those cancer typically presents. Thousands of patients like Mr. Shepard have been confronting gut-wrenching options, delays in treatment and potentially bleaker futures.
Oncologists are concerned that the alternatives to two crucial chemotherapy drugs are far less effective in treating certain cancers, and are sometimes more toxic. The backup therapies or lack thereof, they say, pose particularly troubling prospects for patients with ovarian, testicular, breast, lung and head and neck cancers.
There are few, if any, signs that the shortage will ease anytime soon. A plant that was a main producer of the more popular drugs shut down late last year and has not reopened, depleting its stock. The easing of restrictions on imported drugs from China this month has provided some relief, but doctors said the influx has yet to make much of a dent. Some companies that sell the medications are projecting that the shortage will last through the fall or later.
So far, neither a group of experts organized by the Biden administration nor prominent medical organizations have found a way to avoid rationing the crucial chemo drugs.
To bridge the gaps, some doctors are extending care intervals and skimming precious milliliters to stretch doses. Others are turning to a strategy of surgery first and chemo later, banking on a resumption of supplies.
One of the nation’s top cancer care groups, the American Society of Clinical Oncology, is now advising doctors with low quantities of the medications to administer them to patients with a shot at a cure — and to deny them to patients with recurrent or widely spread disease.
“We’re in a situation where patients are being left behind, and we’re really worried survival could be affected by the chemotherapy shortage,” said Dr. Angeles Alvarez Secord, president of the Society of Gynecologic Oncology and a professor at Duke University School of Medicine.
Two main chemotherapy drugs, cisplatin and carboplatin, are deployed as frontline medicines in cocktails used to shrink or eliminate tumors. More than a dozen cancer drugs are also officially in short supply, as well as hundreds of other medications, including antibiotics and sterile injectable fluids. Still, doctors predict that the absence of the powerful chemotherapies may hurt patients most.
Cisplatin and carboplatin are inexpensive: They cost $15 and $23 per vial, according to the U.S. Pharmacopeia, a nonprofit aimed at medication safety and supply. But manufacturing the drugs requires a reliable supply of platinum, a metal used, as well as a sterile plant and special controls to protect workers from the drugs’ toxic effects. As a result, few companies make them.
The most recent shortages of these widely used drugs occurred when a leading manufacturer, Intas Pharmaceuticals, shut down production in December after the Food and Drug Administration had performed a surprise inspection at its plant in Ahmedabad, India. The U.S. agency issued a report that said employees were shredding, tearing and pouring acid on quality control records and noted a “cascade of failure” at the site.
The company’s subsidiary, Accord Healthcare, in Durham, North Carolina, said recently that it was still making improvements at the plant that were needed to restart production.
By this spring, the effects of the Intas shutdown were deeply felt. A survey by the National Comprehensive Cancer Network of academic treatment centers released earlier this month found that 93 percent of the 27 centers that responded were experiencing a carboplatin shortage. As a result, 36 percent of them reported altering treatments for their patients, resorting to lower doses and longer intervals between therapies.
At cCare Cancer Center in Fresno, Calif., where Mr. Shepard receives care for his vocal cord cancer, efforts to stretch supply have given way to sporadic availability. For the last six weeks, vials of the platinum drugs have been unavailable roughly half of the time, an oncologist, Dr. Ravi Rao, said.
He said Mr. Shepard’s odds of a cure without the drugs would fall from roughly 90 percent to about 45 percent. Fortunately, Mr. Shepard said, the drugs have been available for the first two of seven treatments.
Patients with ovarian cancer are facing the worst outlook, Dr. Rao said, because of how common the disease is and how central the platinum drugs have been in tackling it for decades. Without those drugs, one patient with extensive ovarian cancer has odds of survival that fall to the single digits from about 30 percent, he said.
“This shortage will lead to people dying,” said Dr. Rao, who is also a board member of the Community Oncology Alliance. “There’s just no way around it. You cannot remove these lifesaving drugs and not have bad outcomes.”
Others who face heightened threats are patients with testicular cancer, because cisplatin has a known record of curing even advanced cases, said Dr. Julie Gralow, the ASCO chief medical officer, in her testimony to a House subcommittee earlier this month.
“This is critical, impacting maybe as many as half a million Americans with just these two drugs,” Dr. Gralow said.
For Florida Cancer Specialists, with more than 90 sites, the shortage initially meant conserving 10 to 15 percent of a patient’s dose to stretch stock, said Dr. Lucio Gordan, president of the practice.
That was not enough, so doctors began to only give the drugs to patients with a chance at a cure or those enrolled in clinical trials. The practice found some products at vastly inflated prices — apparent price gouging — but bought them anyway.
Still, by May, the practice was without carboplatin for 12 days and cisplatin for eight days, Dr. Gordan said.
Arias Pitts, 33, who was diagnosed with an aggressive breast cancer in April, encountered the shortage when she arrived to begin treatment on May 16. The carboplatin her doctor had ordered for the first of six rounds of chemotherapy was not available.
“Of course I had questions and concerns,” said Ms. Pitts, an academic adviser at the University of South Florida and a single mother of a 4-year-old. She added: “It’s stressful.”
The F.D.A. has taken steps to ease the shortage. It oversaw the testing and release of batches of the platinum drugs manufactured by Intas in India that were made before the shutdown, but that stock has now been exhausted.
It is also temporarily allowing Qilu Pharmaceuticals, based in China, to ship its cisplatin to the United States.
Jordan Berman, a vice president of Apotex Pharmaceuticals, a Toronto company importing the Qilu drugs, said it received shipments of cisplatin on June 6 and began routing them through major U.S. distributors.
Oncologists and supply chain experts said there was little data so far to gauge the effect the imports would have. About 600 vials of cisplatin from China arrived at Florida Cancer Specialists earlier this month, Dr. Gordan said. But that was not enough for the practice to resume offering the drugs to patients with advanced or recurrent cancers.
“It’s about six days of treatment for us,” Dr. Gordan said. “We’re scrambling.”
Studies in the 1980s and 90s showed that the platinum drugs were a vast improvement over existing treatments, performing best in combination with other drugs and doubling the response rates for ovarian and head and neck cancer. The platinum drugs pushed the five-year survival rate for testicular cancer to 95 percent from roughly 10 percent.
While newer immunotherapy treatments have improved the outcome for patients with certain types of cancer, like melanoma, oncologists also include them in cocktails with the platinum drugs to extend their lives and enhance the potential for survival.
“In general, we haven’t seen these home runs in cancer” in recent years, said Dr. Mikkael Sekeres, a University of Miami oncologist and former F.D.A. oncology adviser.
Oncologists advising the field amid the current shortages have urged those treating early-stage lung cancer patients to send them to a center that has the drugs, noting, “there are no equally effective alternatives.”
Dr. Evan Myers, a Duke University researcher in the obstetrics and gynecology department, said he was planning to measure the effects of the shortages. One study of a different medication shortage affecting children and adolescents with Hodgkin’s lymphoma found that the substitute drug was “significantly less effective,” and reduced the survival rate for the young people who received the backup treatment.
Dr. Myers said this year’s shortages would, at a minimum, likely have an effect on the quality of life for people undergoing treatment. “They’re going to be waiting for the other shoe to drop,” he said.
Doctors are also struggling with how to convey such devastating news, said Dr. Prasanthi Ganesa, medical director of The Center for Cancer and Blood Disorders in Fort Worth. Her practice is looking at each case individually, but is also prioritizing crucial doses for patients who could potentially be cured.
“I can imagine a patient listening to this and saying, ‘You know, I am trying to live longer, that is my priority. So I need that drug, doc,’” she said. “We feel really helpless.”
The situation demands action, said Dr. Karen Knudsen, chief executive of the American Cancer Society. The White House and Congress, which have discussed the problem, have advanced few concrete solutions.
“The necessity for a durable solution is growing greater by the day,” Dr. Knudsen said, adding, “Patients are left hanging.”

Health
Better Than Ozempic? Doctors Say These Medications Are Better for Weight Loss Than the Popular Semaglutide

Sign Up
Create a free account to access exclusive content, play games, solve puzzles, test your pop-culture knowledge and receive special offers.
Already have an account? Login
Forgot your password?
Get back to the Sign In
Use left and right arrow keys to navigate between menu items.
Use escape to exit the menu.
Health
Honeybees can detect lung cancer, researchers say
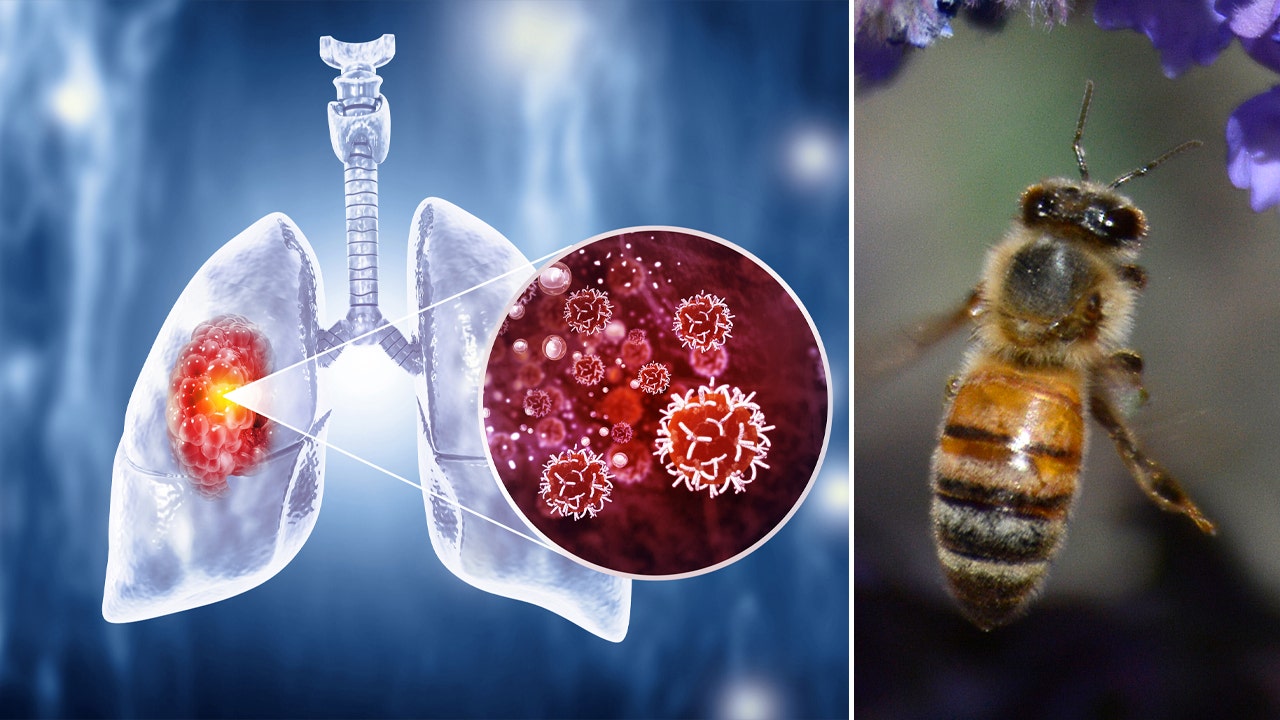
What happens when you pair honeybees and halitosis? Potentially a life-saving new method to screen for cancer, according to one study.
Researchers at Michigan State University have learned that honeybees can detect chemicals associated with lung cancer in human breath. The insects were able to sniff out human lung cancer biomarkers with a remarkable 82% success rate, according to a study published in the journal Biosensors and Bioelectronics.
“These results indicate that the honeybee olfactory system can be used as a sensitive biological gas sensor to detect human lung cancer,” the study authors wrote.
“Insects have an amazing sense of smell the same way dogs do,” said MSU professor Debajit Saha, according to an MSU news release.
YOUNG VAPER WHO REQUIRED DOUBLE LUNG TRANSPLANT SHARES WARNINGS AS E-CIGARETTE SALES RISE
A honeybee drinking nectar from a flower in Markham, Ontario, Canada. (Creative Touch Imaging Ltd./NurPhoto via Getty Images)
Saha, an assistant professor in the College of Engineering and MSU’s Institute for Quantitative Health Science and Engineering, sought to determine whether honeybees could distinguish chemicals in a healthy person’s breath from that of someone sick with lung cancer.
His team developed a “recipe” for a synthetic breath mixture that contained six compounds present in the breath of someone with cancer and a synthetic “healthy” breath mixture.
“It took a steady hand to create the recipe,” said Elyssa Cox, Saha’s former lab manager. “We tested the synthetic lung cancer versus healthy human breath mixtures on approximately 20 bees.”
The researchers placed each live bee in a custom 3D-printed harness and attached a tiny electrode to its brain to measure activity.
SOME BREAST CANCER PATIENTS COULD BE AT RISK OF ANOTHER TYPE OF CANCER, STUDY REVEALS
Lung cancer is the leading cause of cancer death worldwide. An estimated 235,580 people will be diagnosed with lung cancer in 2024 in the U.S., according to the Lung Cancer Research Foundation. (Mohammed Haneefa Nizamudeen/iStock)
“We pass those odors on to the antenna of the honeybees and recorded the neural signals from their brain,” said Saha. “We see a change in the honeybee’s neural firing response.”
The researchers found that the bees were able to detect the cancer-indicating compounds even in small amounts.
“The honeybees detected very small concentrations; it was a very strong result,” said Saha. “Bees can differentiate between minute changes in the chemical concentrations of the breath mixture, which is in the parts per 1 billion range.”
The bees also could tell the difference between the synthetic lung cancer breath and healthy breath.
UK TO INTRODUCE BILL TO PHASE OUT LEGAL SALE OF TOBACCO

A honey bee visits a blooming catmint plant growing in Santa Fe, New Mexico. (Robert Alexander/Getty Images)
Scientists hope this research will lead to the development of a sensor based on a honeybee brain that can be used to test human breath for the presence of lung cancer.
“What’s amazing is the honeybees’ ability to not only detect cancer cells, but also distinguish between cell lines of various types of lung cancer,” said Autumn McLane-Svoboda, a graduate student on Saha’s team. “The future implications for this are huge, as our sensor could allow for patients to receive specific cancer diagnoses quickly, which is imperative for correct treatment routes.”
Lung cancer is the leading cause of cancer death worldwide. An estimated 235,580 people will be diagnosed with lung cancer in 2024 in the U.S., according to the Lung Cancer Research Foundation.
Smoking is the leading risk factor for lung cancer and is responsible for 80% of lung cancer deaths.
Early detection of high-risk lung cancer can reduce the chance of death by up to 20%.
Health
COVID vaccine companies told to focus on KP.2 variant for fall shots, per FDA announcement

The U.S. Food and Drug Administration (FDA) has recommended that COVID vaccine manufacturers update their formulas for fall doses, in an attempt to target the KP.2 strain of the JN.1 variant.
The Thursday announcement came just a week after the agency’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted to recommend a “monovalent JN.1-lineage vaccine” at its June 5 meeting.
As of the end of March 2024, the KP.2 variant was responsible for just 4% of infections in the U.S., according to the COVID Data Tracker from the Centers for Disease Control and Prevention (CDC).
CDC WARNS OF ‘DUAL MUTANT’ FLU STRAIN THAT COULD EVADE ANTIVIRAL DRUGS: ‘NEED TO CLOSELY MONITOR’
Meanwhile, over 50% of infections at that time were attributed to its parental strain, JN.1.
Just a few weeks later, KP.2 is now the cause of around 28% of infections, while the JN.1 variants have largely dropped in prevalence, the tracker shows.
The U.S. Food and Drug Administration has recommended that COVID vaccine manufacturers update their formulas for fall doses, in an attempt to target the KP.2 strain of the JN.1 variant. (iStock)
Dr. Marc Siegel, clinical professor of medicine at NYU Langone Medical Center and a Fox News medical contributor, recently spoke with Dr. Peter Marks, director of the Center for Biologics Evaluation and Research (CBER) at the Food and Drug Administration, about the new vaccine formulations.
“It makes sense to target the KP.2 strain because it is becoming the predominant strain — it is surging in California and will spread across the country,” Siegel told Fox News Digital.

KP.2 is now the cause of around 28% of infections, while the JN.1 variants have largely dropped in prevalence, CDC Tracker data shows. (iStock)
The KP.2 strain is “highly immunoevasive,” the doctor warned — which means that immunity from previous variants and subvariants don’t offer much protection.
COVID-FLU COMBO VACCINE SHOWS ‘POSITIVE’ RESULTS IN PHASE 3 TRIALS, MODERNA SAYS: A ‘TWO-FOR’ OPTION
“On the other hand, the vaccine will cause a production of immune cells and antibodies that will continue to protect you against previous variants and subvariants,” Siegel added.
The updated vaccine is especially important for high-risk groups, those who have chronic illnesses, the elderly and anyone who comes in contact with them, according to doctors. (iStock)
It is especially important for high-risk groups, those who have chronic illnesses, the elderly and anyone who comes in contact with them, according to the doctor.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
In a statement to Fox News Digital, vaccine maker Novavax — which makes protein-based vaccines — said the company “just filed” its application for a JN.1 COVID vaccine.
A vial of the Phase 3 Novavax coronavirus vaccine is seen ready for use in the trial at St. George’s University hospital in London, on Oct. 7, 2020. (AP Photo/Alastair Grant, File)
“Novavax’s updated JN.1 COVID-19 vaccine is active against current circulating strains, including KP.2 and KP.3,” the company said in a press release.
“The submission is in line with guidance from the U.S. FDA, European Medicines Agency (EMA) and the World Health Organization (WHO) to target the JN.1 lineage this fall.”
For more Health articles, visit www.foxnews/health.
Fox News Digital reached out to Pfizer and Moderna — both of which produce mRNA-based vaccines — requesting comment on their plans for fall formulations.
-

 News1 week ago
News1 week agoIsrael used a U.S.-made bomb in a deadly U.N. school strike in Gaza
-

 World1 week ago
World1 week agoFrance to provide Ukraine with its Mirage combat aircraft
-

 World1 week ago
World1 week agoWorld leaders, veterans mark D-Day’s 80th anniversary in France
-

 World1 week ago
World1 week agoRussia-Ukraine war: List of key events, day 833
-

 Movie Reviews1 week ago
Movie Reviews1 week agoInsane Like Me? – Review | Vampire Horror Movie | Heaven of Horror
-

 News1 week ago
News1 week agoNonprofit CFO Accused of 'Simply Astonishing' Fraud
-

 Politics1 week ago
Politics1 week agoGeorge Clooney called White House to complain about Biden’s criticism of ICC and defend wife’s work: report
-

 Politics1 week ago
Politics1 week agoNewson, Dem leaders try to negotiate Prop 47 reform off California ballots, as GOP wants to let voters decide















