Science
Sex, radiation and mummies: How farms are fighting a pesky almond moth without pesticides

In a windowless shack on the far outskirts of Fresno, an ominious red glow illuminates a lab filled with X-ray machines, shelves of glowing boxes, a quietly humming incubator and a miniature wind tunnel.
While the scene looks like something straight out of a sci-fi movie, its actually part of an experimental program to prevent a damaging almond pest from successfully mating.
A moth trap hangs from the branch of an almond tree.
(Gary Kazanjian / For The Times)
With California almond growers reeling from dropping nut prices and rising costs, the pests have only added to their woes.
Every year, the navel orangeworm eats through roughly 2% of California’s almonds before they can make it to grocery store shelves. Last year, it was almost double that.
While that might seem small, if you do the math “it’s going to be a lot of millions of dollars lost to this pest,” said David Haviland, a Kern County farm advisor with University of California Agriculture and Natural Resources. “And that’s despite the control methods that people use,” he said.
California produces 80% of the world’s almonds, yet in 2022 the production value of the nut fell 34% compared with the previous year.
Scientists say climate change could make the navel orangeworm problem even worse, with hotter temperatures allowing the moths to reproduce even faster. (Despite its name, the insect has largely left citrus farms unbothered and is in fact a moth.)
Traditionally, nut farmers have tackled the insect with chemical pesticides, or by destroying “mummies” — almonds left over after harvest. Mummies are a favorite winter shelter for the bugs.
However, research is increasingly showing that chemical pesticides are not only harmful to the environment but to people as well. One new study found that the impact of nearby pesticide use on cancer incidence “may rival that of smoking.”
“When you have to don a spacesuit, basically, to apply something, you’re definitely thinking, ‘This is not good,’” said Houston Wilson, an entomologist with UC ANR’s Kearney Agricultural Research and Extension Center and the mastermind behind the sci-fi shack.
“Across the board, folks want to get away from chemical controls,” he said.
So farmers and researchers have been searching for other non-pesticide alternatives.
Removing almost every last mummy from every tree in an orchard can be effective, but since it must be done manually, it can become too expensive and complex for some growers.
Another tactic that’s been used since around 2010 is to cover orchards with disorienting levels of sex pheromones to confuse horny moths — a technique known as “mating disruption.”
But with limited budgets and climate change threatening to make the pest situation worse, researchers are studying another yet-to-be-proven approach: sterilizing almost a million moths a day with radiation and dropping them out of planes.

Houston Wilson looks over trapped moths at the Kearney Agricultural Research and Extension Center in Parlier, Calif.
(Gary Kazanjian / For The Times)
The idea behind the technique is that by flooding orchards with sterilized insects, they will mate with fertile insects and produce no offspring, reducing the overall population.
The simplest way to sterilize the bugs is to use radiation. Since their reproductive genes tend to mutate faster, the right dose can leave them relatively unfazed but unable to reproduce.
At the request of almond and pistachio farmers, the California Department of Food and Agriculture has been working with the Animal and Plant Health Inspection Service of the U.S. Department of Agriculture since 2018 to source sterilized moths from a Phoenix lab.
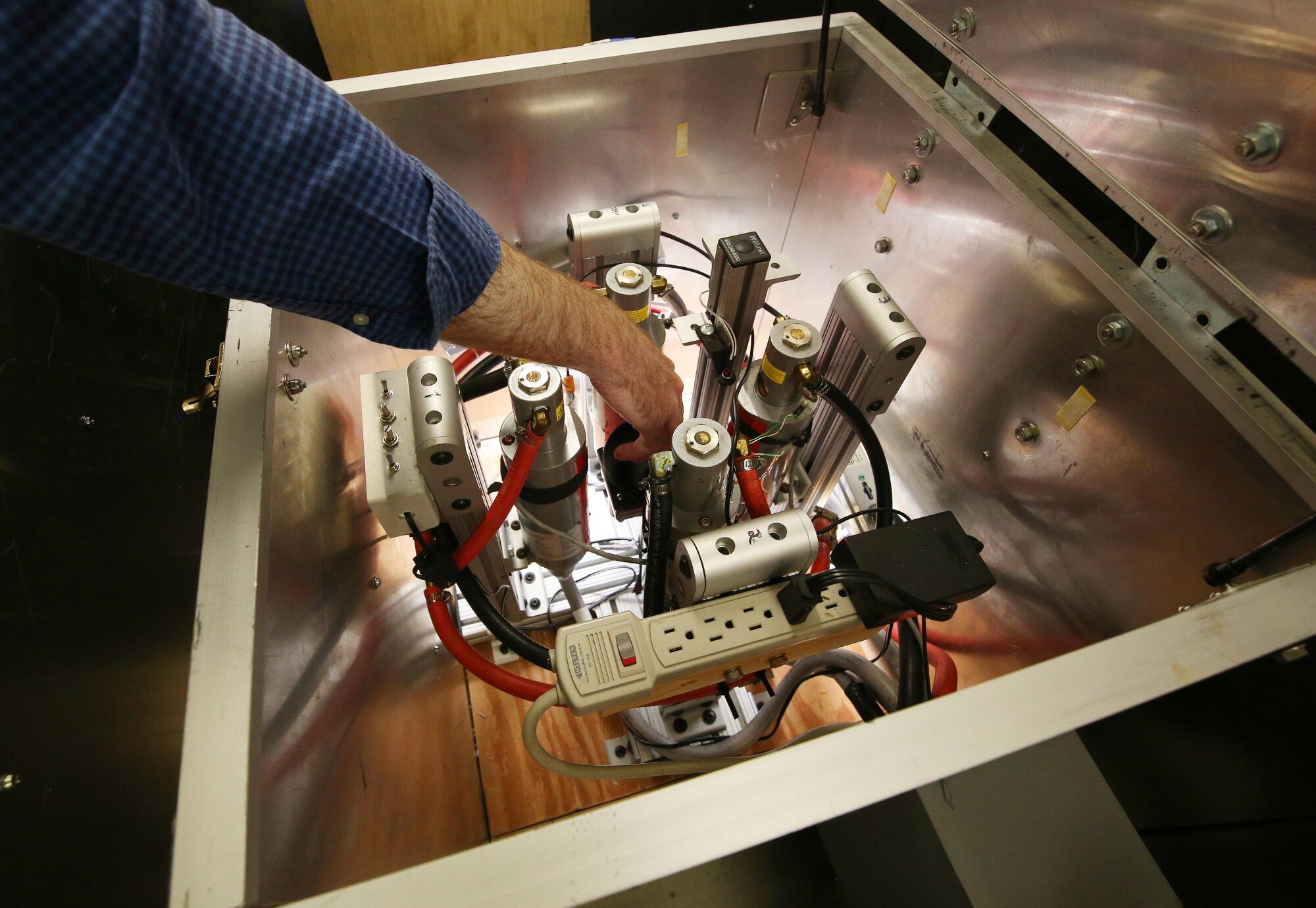
An X-ray machine designed to sterilize moths is shown at the Kearney Agricultural Research and Extension Center.
(Gary Kazanjian / For The Times)
The lab sterilizes about 750,000 bugs per day, then chills the moths to put them to sleep and ships them off to California. The bugs are dropped from an airplane hundreds of feet in the air. Often too sleepy to fly, the insects crash into the hard ground or almond trees.
From there, the survivors have only one job: have sex.
Through this test program, the USDA hopes to perfect the best ways to get moths to reproduce in the lab and give them the right dose of radiation that will sterilize them but not severely injure or disorient them.
The program has yet to put a significant dent in the moth population, though, because they can’t produce enough sterile bugs.
Right now, researchers are only finding a couple of sterile insects in traps for every hundred wild fertile moths. For the technique to be effective, they’ll need to deploy dozens of sterile bugs for every wild one.

Anisa Bel Guzman counts moths at the Kearney Agricultural Research and Extension Center in Parlier, Calif.
(Gary Kazanjian / For The Times)
Matthew Aubuchon, national policy manager at the USDA’s Animal and Plant Health Inspection Service, estimated that the Phoenix facility could produce up to 8 million moths per day with enough staff working around the clock.
While opening more facilities in California would help, the program uses cobalt to produce high-energy radiation to sterilize the bugs — which is expensive and requires the lab to take extensive safety and security measures.
Wilson’s sci-fi shack at Kearney might hold a solution that is cheaper and easier to scale.
Instead of using cobalt or other radioactive materials, Wilson’s team uses an X-ray machine to irradiate the pests. (Unlike a radioactive substance, an X-ray machine will not emit radiation when it is turned off.)
Then, the team puts their X-rayed bugs and the sterilized insects from Phoenix through a series of tests to determine which methods produce the healthiest, sterile moths.
The tests include gluing moths to the end of a stick suspended in the air. The stick rotates like a carousel as the moths flutter around and researchers record how well they can fly.
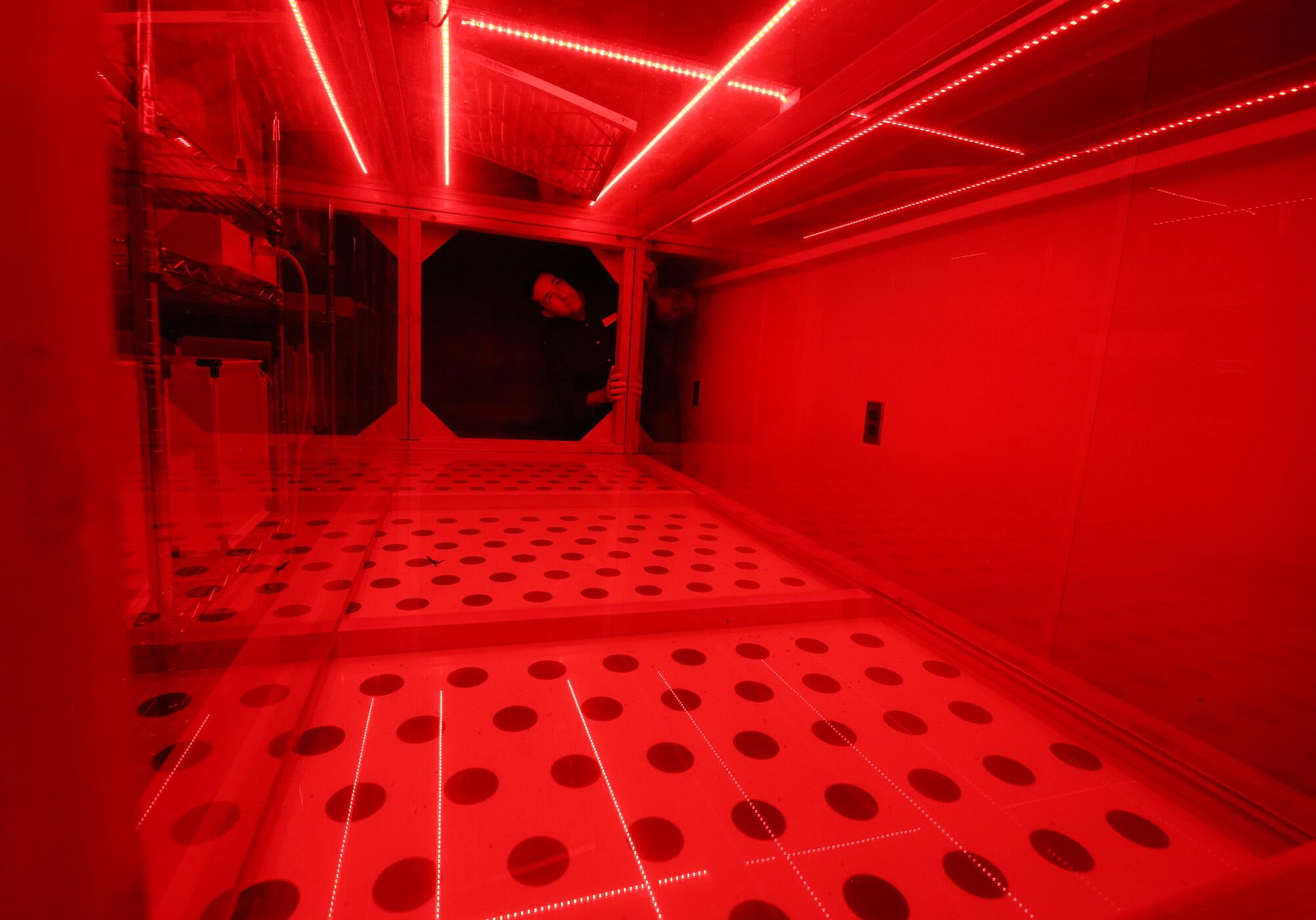
Houston Wilson looks into an insect wind tunnel as researchers look for innovative ways to manage an invasive almond pest.
(Gary Kazanjian / For The Times)
The researchers also place moths in a wind tunnel and release sex pheromones to see if the excited bugs are able to locate the smell. (Unfortunately for the insects, there are no potential mates at the end of the tunnel.)
While the team doesn’t yet produce enough X-rayed moths to test them in a full-blown almond orchard, they do send the Phoenix moths into their final test: releasing them into their seven-acre almond farm on the Kearney campus to see how good they are at actually finding fertile moths to mate with.

Houston Wilson looks over a navel orangeworm trap in an almond field in Parlier.
(Gary Kazanjian / For The Times)
The researchers at Kearney may be in a race against time, however.
Scientists say it’s possible that climate change will continue to tip the weather in the moths’ favor. The metabolism of navel orangeworms — like many agricultural pests — is tied to temperature. The hotter it is, the faster they grow and reproduce.
A 2021 study found that the moths, which can have life cycles as short as just one month, may be able to squeeze in another generation each summer before holing up in nuts for the winter.
“For each additional generation, their population is increasing at an exponential rate,” said Tapan Pathak, an author on the study and a professor at UC Merced.
“If this additional generation is coinciding with … harvest,” Pathak said, “then they become unmarketable. That’s a huge economic loss.”
However, the food web is complicated, and just because the warmer weather benefits the moths on paper doesn’t mean the moths will end up on top.

Every year, navel orangeworms eat through roughly 2% of California’s almonds before they can make it to grocery store shelves.
(Gary Kazanjian / For The Times)
“Navel orangeworm could be a nightmare … but it could also become less of a problem because all the things that eat it benefit more from the heat than the navel orangeworm,” said Haviland. “The crystal ball is certainly not clear enough to know what will happen.”
Researchers stress that successful pest control will require multiple measures.
“What we’ve learned through integrated pest management is that the timing of one or staggering of different approaches together yields results for the growers,” said Aubuchon.
The tried-and-true non-pesticide method growers have been using since the moths’ unannounced arrival in the 1940s is to simply ensure all the almonds are either harvested or destroyed by the time winter arrives.
But for this method to be effective, there must be no more than two almonds left on every tree in an orchard. This can be hard to achieve in wet weather.
Rain makes almond branches soggy and flexible, which makes it hard to snap nuts off using an industrial shaker. Damp earth can also make it difficult for machines to get close to the trees.
Instead, workers must use poles to knock almonds off manually. As effective as this is, increasing labor costs mean some farms just can’t afford it.

Anisa Bel Guzman counts moths at the Kearney Agricultural Research and Extension Center in Parlier.
(Gary Kazanjian / For The Times)
While researchers say the sterile insect technique still has a lot of hurdles to clear before it will be widely effective, they say it holds great promise.
“You’re literally managing a pest by preventing it from being born in the first place,” said Haviland of both sterile insect technique and pheromone mating disruption. “To think that something like that was possible 10 or 15 years ago — nobody could imagine that growers would be using such innovative techniques as those.”

Science
A virus without a vaccine or treatment is hitting California. What you need to know

A respiratory virus that doesn’t have a vaccine or a specific treatment regimen is spreading in some parts of California — but there’s no need to sound the alarm just yet, public health officials say.
A majority of Northern California communities have seen high concentrations of human metapneumovirus, or HMPV, detected in their wastewater, according to data from the WastewaterScan Dashboard, a public database that monitors sewage to track the presence of infectious diseases.
A Los Angeles Times data analysis found the communities of Merced in the San Joaquin Valley, and Novato and Sunnyvale in the San Francisco Bay Area have seen increases in HMPV levels in their wastewater between mid-December and the end of February.
HMPV has also been detected in L.A. County, though at levels considered low to moderate at this point, data show.
While HMPV may not necessarily ring a bell, it isn’t a new virus. Its typical pattern of seasonal spread was upended by the COVID-19 pandemic, and its resurgence could signal a return to a more typical pre-coronavirus respiratory disease landscape.
Here’s what you need to know.
What is HMPV?
HMPV was first detected in 2001, according to the U.S. Centers for Disease Control and Prevention. It’s transmitted by close contact with someone who is infected or by touching a contaminated surface, said Dr. Neha Nanda, chief of infectious diseases and hospital epidemiologist for Keck Medicine of USC.
Like other respiratory illnesses, such as influenza, HMPV spreads and is more durable in colder temperatures, infectious-disease experts say.
Human metapneumovirus cases commonly start showing up in January before peaking in March or April and then tailing off in June, said Dr. Jessica August, chief of infectious diseases at Kaiser Permanente Santa Rosa.
However, as was the case with many respiratory viruses, COVID disrupted that seasonal trend.
Why are we talking about HMPV now?
Before the pandemic hit in 2020, Americans were regularly exposed to seasonal viruses like HMPV and developed a degree of natural immunity, August said.
That protection waned during the pandemic, as people stayed home or kept their distance from others. So when people resumed normal activities, they were more vulnerable to the virus. Unlike other viruses, there isn’t a vaccine for human metapneumovirus.
“That’s why after the pandemic we saw record-breaking childhood viral illnesses because we lacked the usual immunity that we had, just from lack of exposure,” August said. “All of that also led to longer viral seasons, more severe illness. But all of these things have settled down in many respects.”
In 2024, the national test positivity for HMPV peaked at 11.7% at the end of March, according to the National Respiratory and Enteric Virus Surveillance System. The following year’s peak was 7.15% in late April.
So far this year, the highest test positivity rate documented was 6.1%, reported on Feb. 21 — the most recent date for which complete data are available.
While the seasonal spread of viruses like HMPV is nothing new, people became more aware of infectious diseases and how to prevent them during the pandemic, and they’ve remained part of the public consciousness in the years since, August and Nanda said.
What are the symptoms of HMPV?
Most people won’t go to the doctor if they have HMPV because it typically causes mild, cold-like symptoms that include cough, fever, nasal congestion and sore throat.
HMPV infection can progress to:
- An asthma attack and reactive airway disease (wheezing and difficulty breathing)
- Middle ear infections behind the ear drum
- Croup, also known as “barking” cough — an infection of the vocal cords, windpipe and sometimes the larger airways in the lungs
- Bronchitis
- Fever
Anyone can contract human metapneumovirus, but those who are immunocompromised or have other underlying medical conditions are at particular risk of developing severe disease — including pneumonia. Young children and older adults are also considered higher-risk groups, Nanda said.
What is the treatment for HMPV?
There is no specified treatment protocol or antiviral medication for HMPV. However, it’s common for an infection to clear up on its own and treatment is mostly geared toward soothing symptoms, according to the American Lung Assn.
A doctor will likely send you home and tell you to rest and drink plenty of fluids, Nanda said.
If symptoms worsen, experts say you should contact your healthcare provider.
How to avoid contracting HMPV
Infectious-disease experts said the best way to avoid contracting HMPV is similar to preventing other respiratory illnesses.
The American Lung Assn.’s recommendations include:
- Wash your hands often with soap and water. If that’s not available, clean your hands with an alcohol-based hand sanitizer.
- Clean frequently touched surfaces.
- Crack open a window to improve air flow in crowded spaces.
- Avoid being around sick people if you can.
- Avoid touching your eyes, nose and mouth.
Assistant data and graphics editor Vanessa Martínez contributed to this report.
Science
After rash of overdose deaths, L.A. banned sales of kratom. Some say they lost lifeline for pain and opioid withdrawal

Nearly four months ago, Los Angeles County banned the sale of kratom, as well as 7-OH, the synthetic version of the alkaloid that is its active ingredient. The idea was to put an end to what at the time seemed like a rash of overdose deaths related to the drug.
It’s too soon to tell whether kratom-related deaths have dissipated as a result — or, really, whether there was ever actually an epidemic to begin with. But many L.A. residents had become reliant on kratom as something of a panacea for debilitating pain and opioid withdrawal symptoms, and the new rules have made it harder for them to find what they say has been a lifesaving drug.
Robert Wallace started using kratom a few years ago for his knees. For decades he had been in pain, which he says stems from his days as a physical education teacher for the Glendale Unified School District between 1989 and 1998, when he and his students primarily exercised on asphalt.
In 2004, he had arthroscopic surgery on his right knee, followed by varicose vein surgery on both legs. Over the next couple of decades, he saw pain-management specialists regularly. But the primary outcome was a growing dependence on opioid-based painkillers. “I found myself seeking doctors who would prescribe it,” he said.
He leaned on opioids when he could get them and alcohol when he couldn’t, resulting in a strain on his marriage.
When Wallace was scheduled for his first knee replacement in 2021 (he had his other knee replaced a few years later), his brother recommended he take kratom for the post-surgery pain.
It seemed to work: Wallace said he takes a quarter of a teaspoon of powdered kratom twice a day, and it lets him take charge of managing his pain without prescription painkillers and eases harsh opiate-withdrawal symptoms.
He’s one of many Angelenos frustrated by recent efforts by the county health department to limit access to the drug. “Kratom has impacted my life in only positive ways,” Wallace told The Times.
For now, Wallace is still able to get his kratom powder, called Red Bali, by ordering from a company in Florida.
However, advocates say that the county crackdown on kratom could significantly affect the ability of many Angelenos to access what they say is an affordable, safer alternative to prescription painkillers.
Kratom comes from the leaves of a tree native to Southeast Asia called Mitragyna speciosa. It has been used for hundreds of years to treat chronic pain, coughing and diarrhea as well as to boost energy — in low doses, kratom appears to act as a stimulant, though in higher doses, it can have effects more like opioids.
Though advocates note that kratom has been used in the U.S. for more than 50 years for all sorts of health applications, there is limited research that suggests kratom could have therapeutic value, and there is no scientific consensus.
Then there’s 7-OH, or 7-Hydroxymitragynine, a synthetic alkaloid derived from kratom that has similar effects and has been on the U.S. market for only about three years. However, because of its ability to bind to opioid receptors in the body, it has a higher potential for abuse than kratom.
Public health officials and advocates are divided on kratom. Some say it should be heavily regulated — and 7-OH banned altogether — while others say both should be accessible, as long as there are age limitations and proper labeling, such as with alcohol or cannabis.
In the U.S., kratom and 7-OH can be found in all sorts of forms, including powder, capsules and liquids — though it depends on exactly where you are in the country. Though the Food and Drug Administration has recommended that 7-OH be included as a Schedule 1 controlled substance under the Controlled Substances Act, that hasn’t been made official. And the plant itself remains unscheduled on the federal level.
That has left states, counties and cities to decide how to regulate the substances.
California failed to approve an Assembly bill in 2024 that would have required kratom products to be registered with the state, have labeling and warnings, and be prohibited from being sold to anyone younger than 21.
It would also have banned products containing synthetic versions of kratom alkaloids. The state Legislature is now considering another bill that basically does the same without banning 7-OH — while also limiting the amount of synthetic alkaloids in kratom and 7-OH products sold in the state.
“Until kratom and its pharmacologically active key ingredients mitragynine and 7-OH are approved for use, they will remain classified as adulterants in drugs, dietary supplements and foods,” a California Department of Public Health spokesperson previously told The Times.
On Tuesday, California Gov. Gavin Newsom announced that the state’s efforts to crack down on kratom products has resulted in the removal of more than 3,300 kratom and 7-OH products from retail stores. According to a news release from the governor’s office, there has been a 95% compliance rate from businesses in removing the products.
(Los Angeles Times photo illustration; source photos by Getty Images)
Newsom has equated these actions to the state’s efforts in 2024 to quash the sale of hemp products containing cannabinoids such as THC. Under emergency state regulations two years ago, California banned these specific hemp products and agents with the state Department of Alcoholic Beverage Control seized thousands of products statewide.
Since the beginning of 2026, there have been no reported violations of the ban on sales of such products.
“We’ve shown with illegal hemp products that when the state sets clear expectations and partners with businesses, compliance follows,” Newsom said in a statement. “This effort builds on that model — education first, enforcement where necessary — to protect Californians.”
Despite the state’s actions, the Los Angeles County Board of Supervisors is still considering whether to regulate kratom, or ban it altogether.
The county Public Health Department’s decision to ban the sale of kratom didn’t come out of nowhere. As Maral Farsi, deputy director of the California Department of Public Health, noted during a Feb. 18 state Senate hearing, the agency “identified 362 kratom-related overdose deaths in California between 2019 and 2023, with a steady increase from 38 in 2019 up to 92 in 2023.”
However, some experts say those numbers aren’t as clear-cut as they seem.
For example, a Los Angeles Times investigation found that in a number of recent L.A. County deaths that were initially thought to be caused by kratom or 7-OH, there wasn’t enough evidence to say those drugs alone caused the deaths; it might be the case that the danger is in mixing them with other substances.
Meanwhile, the actual application of this new policy seems to be piecemeal at best.
The county Public Health Department told The Times it conducted 2,696 kratom-related inspections between Nov. 10 and Jan. 27, and found 352 locations selling kratom products. The health department said the majority stopped selling kratom after those inspections; there were nine locations that ignored the warnings, and in those cases, inspectors impounded their kratom products.
But the reality is that people who need kratom will buy it on the black market, drive far enough so they get to where it’s sold legally or, like Wallace, order it online from a different state.
For now, retailers who sell kratom products are simply carrying on until they’re investigated by county health inspectors.
Ari Agalopol, a decorated pianist and piano teacher, saw her performances and classes abruptly come to a halt in 2012 after a car accident resulted in severe spinal and knee injuries.
“I tried my best to do traditional acupuncture, physical therapy and hydrocortisone shots in my spine and everything,” she said. “Finally, after nothing was working, I relegated myself to being a pain-management patient.”
She was prescribed oxycodone, and while on the medication, battled depression, anhedonia and suicidal ideation. She felt as though she were in a fog when taking oxycodone, and when it ran out, ”the pain would rear its ugly head.” Agalopol struggled to get out of bed daily and could manage teaching only five students a week.
Then, looking for alternatives to opioids, she found a Reddit thread in which people were talking up the benefits of kratom.
“I was kind of hesitant at first because there’re so many horror stories about 7-OH, but then I researched and I realized that the natural plant is not the same as 7-OH,” she said.
She went to a local shop, Authentic Kratom in Woodland Hills, and spoke to a sales associate who helped her decide which of the 47 strains of kratom it sold would best suit her needs.
Agalopol currently takes a 75-milligram dose of mitragynine, the primary alkaloid in kratom, when necessary. It has enabled her to get back to where she was before her injury: teaching 40 students a week and performing every weekend.
Agalopol believes the county hasn’t done its homework on kratom. “They’re just taking these actions because of public pressure, and public pressure is happening because of ignorance,” she said.
During the course of reporting this story, Authentic Kratom has shut down its three locations; it’s unclear if the closures are temporary. The owner of the business declined to comment on the matter.
When she heard the news of the recent closures, Agalopol was seething. She told The Times she has enough capsules of kratom for now, but when she runs out, her option will have to be Tylenol and ibuprofen, “which will slowly kill my liver.”
“Prohibition is not a public health strategy,” said Jackie Subeck, executive director of 7-Hope Alliance, a nonprofit that promotes safe and responsible access to 7-OH for consumers, at the Feb. 18 Senate hearing. “[It’s] only going to make things worse, likely resulting in an entirely new health crisis for Californians.”
Science
There were 13 full-service public health clinics in L.A. County. Now there are 6

Because of budget cuts, the Los Angeles County Department of Public Health has ended clinical services at seven of its public health clinic sites.
As of Feb. 27, the county is no longer providing services such as vaccinations, sexually transmitted infection testing and treatment, or tuberculosis diagnosis and specialty TB care at the affected locations, according to county officials and a department fact sheet.
The sites losing clinical services are Antelope Valley in Lancaster; the Center for Community Health (Leavy) in San Pedro, Curtis R. Tucker in Inglewood, Hollywood-Wilshire, Pomona, Dr. Ruth Temple in South Los Angeles, and Torrance. Services will continue to be provided by the six remaining public health clinics, and through nearby community clinics.
The changes are the result of about $50 million in funding losses, according to official county statements.
“That pushed us to make the very difficult decision to end clinical services at seven of our sites,” said Dr. Anish Mahajan, chief deputy director of the L.A. County Department of Public Health.
Mahajan said the department selected clinics with relatively lower patient volumes. Over the last month, he said, the department has sent letters to patients about the changes, and referred them to unaffected county clinics, nearby federally qualified health centers or other community providers. According to Mahajan, for tuberculosis patients, particularly those requiring directly observed therapy, public health nurses will continue visiting patients.
Public health clinics form part of the county’s healthcare safety net, serving low-income residents and those with limited access to care. Officials said that about half of the patients the county currently sees across its clinics are uninsured.
Mahajan noted that the clinics were established decades ago, before the Affordable Care Act expanded Medi-Cal coverage and increased the number of federally qualified health centers. He said that as more residents gained access to primary care, utilization at some county-run clinics declined.
“Now that we have a more sophisticated safety net, people often have another place to go for their full range of care,” he said.
Still, the closures have unsettled providers who work closely with local vulnerable populations.
“I hate to see any services that serve our at-risk and homeless community shut down,” said Mark Hood, chief executive of Union Rescue Mission in downtown Los Angeles. “There’s so much need out there, so it always is going to create hardship for the people that actually need the help the most.”
Union Rescue Mission does not receive government funding for its healthcare services, Hood said. The mission’s clinics are open not only to shelter guests, up to 1,000 people nightly, but also to people living on the streets who walk in seeking care.
Its dental clinic alone sees nearly 9,000 patients a year, Hood said.
“We haven’t seen it yet, but I expect in the coming days and weeks we’ll see more people coming through our doors looking for help,” he said. “They’re going to have to find help somewhere.” Hood said women experiencing homelessness are especially vulnerable when preventive care, including sexual and reproductive health services, becomes harder to access.
County officials said staffing impacts so far have been managed through reassignment rather than layoffs. Roughly 200 to 300 positions across the department have been eliminated amid funding cuts, officials said, though many were vacant. About 120 employees whose positions were affected have been reassigned; according to Mahajan, no one has been laid off.
The clinic closures come amid broader fiscal uncertainty. Mahajan said that due to the Trump administration’s “Big Beautiful Bill,” Los Angeles County could lose $2.4 billion over the next several years. That funding, he said, supports clinics, hospitals and community clinic partners now absorbing patients who previously went to the clinics that closed on Feb. 27.
In response, the L.A. County Board of Supervisors has backed a proposed half-cent sales tax measure that would generate hundreds of millions of dollars annually for healthcare and public health services. Voters are expected to consider the measure in June.
-

 World1 week ago
World1 week agoExclusive: DeepSeek withholds latest AI model from US chipmakers including Nvidia, sources say
-

 Wisconsin4 days ago
Wisconsin4 days agoSetting sail on iceboats across a frozen lake in Wisconsin
-

 Massachusetts1 week ago
Massachusetts1 week agoMother and daughter injured in Taunton house explosion
-

 Maryland5 days ago
Maryland5 days agoAM showers Sunday in Maryland
-

 Massachusetts3 days ago
Massachusetts3 days agoMassachusetts man awaits word from family in Iran after attacks
-

 Florida5 days ago
Florida5 days agoFlorida man rescued after being stuck in shoulder-deep mud for days
-

 Denver, CO1 week ago
Denver, CO1 week ago10 acres charred, 5 injured in Thornton grass fire, evacuation orders lifted
-

 Oregon6 days ago
Oregon6 days ago2026 OSAA Oregon Wrestling State Championship Results And Brackets – FloWrestling



















