Health
Stem cell therapy to correct heart failure in children could 'transform lives'

Renowned visionary English physician William Harvey wrote in 1651 about how our blood contains all the secrets of life.
“And so I conclude that blood lives and is nourished of itself and in no way depends on any other part of the body as being prior to it or more excellent,” he wrote. “So that from this we may perceive the causes not only of life in general … but also of longer or shorter life, of sleeping and waking, of skill, of strength and so forth.”
Dr. Kevin Watt, team leader of the Heart Regeneration and Disease Laboratory at the Murdoch Children’s Research Institute (MCRI) in Melbourne, Australia, understands this concept deeply.
STEM CELL RESEARCH SHOWING NEW POSSIBILITIES FOR TREATING INFANT HEART DISEASE
He lives it every day, as he and his fellow researchers study and reprogram the potential of the blood to treat disease, specifically heart failure in children.
Building on the work of Dr. Shinya Yamanaka of Japan, who discovered that specialized cells could be reprogrammed back to immature stem cells, Watt and his collaborators have taken this work several steps further.
During upcoming clinical trials, “large sheets of heart tissue will be stitched into the failing heart,” said Dr. Kevin Watt, team leader of the Heart Regeneration and Disease Laboratory at Murdoch Children’s Research Institute (MCRI) in Melbourne, Australia. (MCRI)
They have used small molecules to turn these new stem cells from the blood into heart cells.
Small heart organoids are developed in the lab — which can then be injected into the failing hearts of children.
BOY FACING BLINDNESS GETS LIFE-CHANGING EYE SURGERY: ‘SUCH A BLESSING’
Relying on the philanthropic support of the Murdoch Institute, the work is progressing rapidly and has been shown to be effective already in mice, pigs and sheep.
“The vision of our research is to develop new therapies that can transform the lives of children with heart failure.”
Clinical trials in humans will be starting soon, and as Dr. Watt told me in an interview from Australia, “Large sheets of heart tissue will be stitched into the failing heart.”
Congenital heart failure as well as side effects of chemotherapy in children will be targets for this miracle therapy. Millions of children around the world suffer daily from these conditions.

Researchers at MCRI are studying and reprogramming the potential of the blood to treat disease, specifically heart failure in children. (MCRI)
Watt said that certain chemotherapy (anthracyclines) have a higher risk of heart failure – up to 15% of the time – and this treatment may be useful to protect the heart.
Watt said, “Heart failure remains an urgent, unmet clinical challenge across the world. While we have made significant advances over several decades in managing the disease, we lack targeted therapies to treat these devastating conditions.”
FAMILY OF CHILD WITH DOWN SYNDROME WENT FROM SHOCK TO GRATITUDE: ‘LOST THE AIR IN MY CHEST’
He added, “More than 500,000 children around the world live with advanced heart failure that requires transplantation. The vision of our research is to develop new therapies that can transform the lives of children with heart failure.”

“More than 500,000 children around the world live with advanced heart failure that requires transplantation. The vision of our research is to develop new therapies that can transform the lives of children with heart failure.” (iStock)
To achieve this, he said, “we use a technology called induced pluripotent stem cells, where we can convert blood or skin cells of patients with heart failure into stem cells that we then turn into heart cells … or even make engineered heart tissues that can be stitched onto the patient’s heart to help it pump.”
The cells that are targeted in the blood are known as peripheral blood mononuclear cells (PBMCs).
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
They are “pushed back in time to an earlier time before they became differentiated into heart or kidney cells,” he said.
Then they can be pushed forward to become healthy heart cells or mutations — or other abnormalities can be corrected.
While the team at the Murdoch Children’s Research Institute is making heart cells from stem cells in the blood for clinical use, it’s also using these stem cells to figure out new drugs to treat heart failure directly.

The team at MCRI in Melbourne (shown above) is pioneering “methods to turn stem cells into miniature heart tissues.” (MCRI)
Said Watt, “Using stem cells from patients with heart failure caused by chemo, we are actively developing new drugs and cell-based treatments that we believe will transform the lives of patients with these conditions … Our research group has pioneered methods to turn these stem cells into miniature heart tissues that can be used to model disease-in-a-dish, to identify new drug targets for the development of new therapies.”
These treatments are personalized and highly expensive, but they’re also highly effective.

“Using stem cells from patients with heart failure caused by chemo, we are actively developing new drugs and cell-based treatments that we believe will transform the lives of patients with these conditions.” (MCRI)
Correcting heart failure in young children is only a few years away from becoming a reality.
It’s a Christmas miracle that relies on the kind of philanthropic support that MCRI is famous for arranging.
“Philanthropic support plays a critical role in accelerating the development of these new, transformative treatments,” said Watt, “and this support will be essential as we work toward bringing stem cell-based precision therapies for heart failure to every child who needs it.”
For more Health articles, visit www.foxnews.com/health
Visit go.fox/MCRI to donate or to learn more about MCRI’s important research.

Health
FDA approves first medication for obstructive sleep apnea, which also promotes weight loss
The first medication for obstructive sleep apnea has been approved by the U.S. Food and Drug Administration (FDA).
On Dec. 20, the FDA announced that the agency has approved Eli Lilly’s Zepbound (tirzepatide) to treat moderate to severe obstructive sleep apnea (OSA) in adults with obesity.
The drug is to be paired with a reduced-calorie diet and increased physical activity, the FDA noted.
SOME SLEEP APNEA PATIENTS SEE IMPROVEMENT WITH NEW BREATHING TOOL
Sally Seymour, M.D., director of the Division of Pulmonology, Allergy and Critical Care in the FDA’s Center for Drug Evaluation and Research in Washington, D.C., applauded the approval in an announcement.
Happy and healthy senior man sleeping deeply on his left side without snoring (iStock)
“Today’s approval marks the first drug treatment option for certain patients with obstructive sleep apnea,” Seymour wrote. “This is a major step forward for patients with obstructive sleep apnea.”
OSA occurs when the upper airway becomes blocked and causes pauses in breathing during sleep, according to the FDA. The condition is more common in people who are overweight or obese.
UNTREATED SLEEP APNEA PRESENTS ‘DISRUPTIVE’ DANGERS TO PEOPLE’S LIVES, INCLUDING HEART ISSUES, SAYS EXPERT
Similar to semaglutide treatments like Ozempic and Wegovy, Zepbound activates receptors of hormones secreted from the intestine (GLP-1 and GIP) to reduce appetite and food intake.
About 30 million people suffer from sleep apnea in the U.S.
Studies show that by reducing body weight, Zepbound “also improves OSA,” the FDA noted.
In a 52-week study, participants treated with Zepbound experienced “statistically significant and clinically meaningful reduction in events of apnea or hypopnea,” and a large share of participants achieved remission or “resolution of symptoms.”

Zepbound improves obstructive sleep apnea by reducing body weight, studies show. (iStock)
Zepbound-treated patients also reported a significant decrease in body weight, the FDA mentioned.
The drug can reportedly cause side effects like nausea, diarrhea, vomiting, constipation, stomach discomfort and pain, injection site reactions, fatigue, allergic reactions (typically fever and rash), burping, hair loss and gastroesophageal reflux disease.
DO WOMEN NEED MORE SLEEP THAN ME? HERE’S WHAT EXPERTS THINK
While Zepbound causes thyroid C-cell tumors in rats, it’s unknown whether it causes these tumors in humans, so it should not be used by patients with a personal or family history of medullary thyroid cancer or with multiple endocrine neoplasia syndrome type 2, per the FDA.
The agency encourages all OSA patients to consult with a doctor before taking Zepbound and to monitor for any complications.

One sleep expert called the FDA’s approval a “promising advancement for the millions of people who suffer from this condition.” (iStock)
Sleep expert Dr. Wendy Troxel, who is a RAND Corporation senior behavioral specialist and licensed clinical psychologist in Utah, called the FDA’s approval a “promising advancement for the millions of people who suffer from this condition.”
“Zepbound promotes weight loss and has been shown to reduce apnea events.”
About 30 million people suffer from sleep apnea in the U.S., Troxel told Fox News Digital.
While the most common treatment for sleep apnea — positive airway pressure (PAP) — is “highly effective” at treating the condition, up to 50% of patients are “non-adherent,” she said.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
“Zepbound promotes weight loss and has been shown to reduce apnea events,” Troxel noted.
“For obese individuals with moderate to severe sleep apnea, this new treatment option may offer an important alternative or adjunctive treatment, particularly for those who struggle with sleep apnea therapies, such as positive airway pressure.”

People with a history of severe allergic reaction to tirzepatide should not use Zepbound, the FDA warned in a news release. (iStock)
Troxel added that treating sleep apnea is “critical not only for the patient’s health and well-being, but also for their bed partners, who are often the ‘hidden casualties’ of untreated sleep apnea, given that loud snoring and gasping for air are primary symptoms,” she said.
For more Health articles, visit www.foxnews.com/health
Dr. William Lu, medical director of Dreem Health in San Francisco, told Fox News Digital that obesity and sleep apnea are “two of the most prevalent health conditions that affect Americans today.”
“We still need to emphasize shifting the diet and health habits of many, but this is a fantastic start.”
“And they go hand in hand,” he said. “For the patients who qualify and have no contraindications, tirzepatide has the opportunity to be a generational medication that can help people lose weight, reduce the severity of sleep apnea and improve overall health.”
“We still need to emphasize shifting the diet and health habits of many, but this is a fantastic start.”
Getting tested for sleep apnea is “critical for your health,” Lu said, and could also be a requirement for getting coverage for the medication.
Fox News Digital reached out to Eli Lilly requesting comment.
Health
First rare human case of bird flu reported in Los Angeles County
The first human case of H5N1, more commonly known as avian influenza or bird flu, has been confirmed in Los Angeles County in California, in connection with an adult who was exposed to livestock.
The Los Angeles County Department of Public Health said the person who contracted bird flu had mild symptoms, was treated with antivirals and is now recovering at home.
While this is the first human case in the county, the public health department said the overall risk of H5 bird flu to the public remains low, adding that there is currently no evidence of person-to-person spread of the virus.
Still, those who are in close contact with the infected person, as well as other workers at the worksite, are being monitored for symptoms and have been offered testing, antiviral prophylaxis and personal protective equipment.
GOVERNOR NEWSOM DECLARES STATE OF EMERGENCY IN CALIFORNIA DUE TO BIRD FLU
The CDC said on Wednesday a patient has been hospitalized with a severe case of H5N1 infection in Louisiana. (Reuters/Dado Ruvic/Illustration/File Photo)
The case is part of an ongoing investigation which involves the county public health department, the California Department of Public Health and the Centers for Disease Control and Prevention.
“People rarely get bird flu, but those who interact with infected livestock or wildlife have a greater risk of infection. This case reminds us to take basic precautions to prevent being exposed,” LA County health officer Muntu Davis said. “People should avoid unprotected contact with sick or dead animals including cows, poultry, and wild birds; avoid consuming raw or undercooked animal products, such as raw milk; and protect pets and backyard poultry from exposure to wild animals.”
Davis also encouraged the public to get the seasonal flu vaccine, which he said can help prevent severe seasonal flu illness and lower the risk of getting seasonal and bird flu infections at the same time if exposed.
BIRD FLU CAUSES DEATHS OF CATS AND ZOO ANIMALS AS VIRUS SPREADS IN US
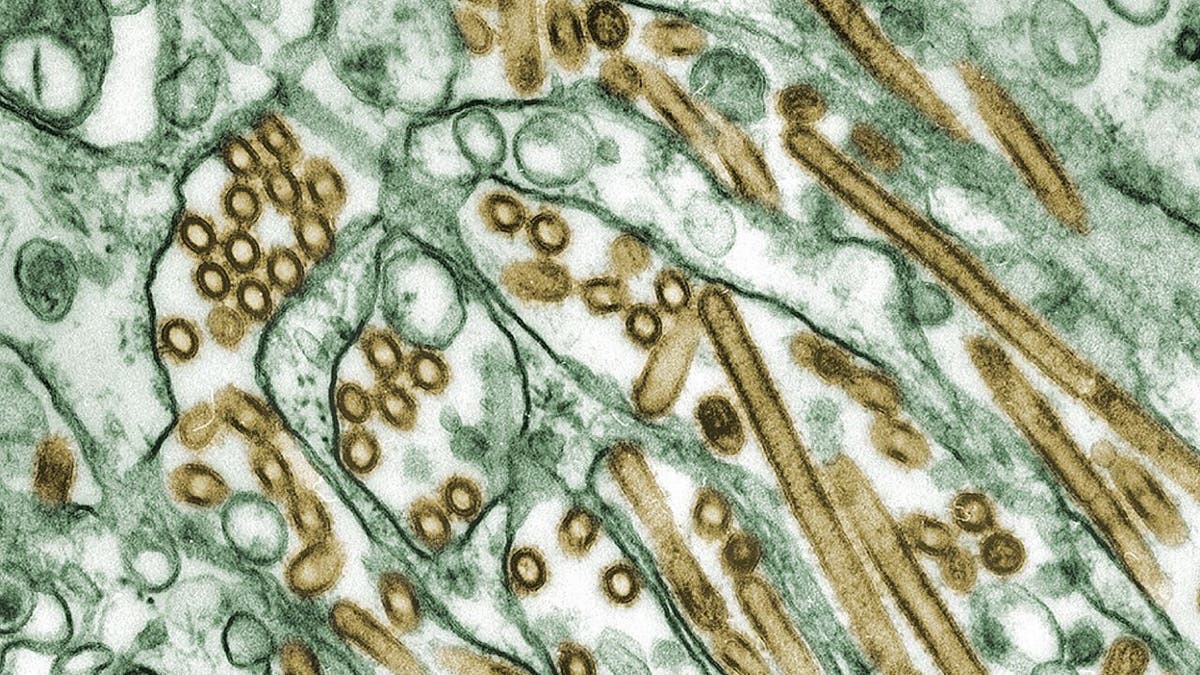
Colorized transmission electron micrograph of Avian influenza A H5N1 viruses (seen in gold) grown in MDCK cells (seen in green). (Smith Collection/Gado/Getty Images)
Bird flu symptoms in humans include eye redness or discharge, fever, cough or difficulty breathing, sore throat, muscle aches, diarrhea and vomiting.
The news comes just days after California Gov. Gavin Newsom declared a state of emergency due to bird flu.
Newsom issued the state of emergency after an outbreak of the virus occurred among dairy cows in Southern California farms.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER

At least two farm workers have been infected with H5N1 bird flu this year. (iStock)
After initially being reported in Texas and Kansas in March, bird flu has been confirmed in cattle across 16 U.S. states, as the Centers for Disease Control and Prevention (CDC) has reported.
“This proclamation is a targeted action to ensure government agencies have the resources and flexibility they need to respond quickly to this outbreak,” Newsom said in a statement.
Also, last week, the first case of severe bird flu was confirmed in a human patient in Louisiana, according to the CDC.
The health agency maintains that “the current public health risk is low,” but said it is “watching the situation carefully.”
Fox News Digital’s Melissa Rudy contributed to this report.
Health
The year in cancer: Advances made in 2024, predictions for 2025
At the beginning of 2024, the American Cancer Society predicted that 2,001,140 new cancer cases and 611,720 cancer deaths would occur in the United States.
Now, as the year draws to a close, experts are looking back and reflecting on the discoveries and advances that have been made in the field of cancer treatment and prevention.
Fox News Digital spoke with four oncologists from the Sarah Cannon Research Institute in Nashville, Tennessee, about the most notable accomplishments of 2024 and what they see on the horizon for 2025.
5 CANCER TYPES WHERE SCREENINGS SAVE THE MOST LIVES
See the answers and questions below.
Krish Patel, MD, director of lymphoma research
Krish Patel, M.D., is director of lymphoma research at Sarah Cannon Research Institute in Nashville, Tennessee. (Sarah Cannon Research Institute )
Q: What do you see as the most important cancer advances in 2024?
A: In the field of lymphomas, we see growing momentum for therapies that use the patient’s own immune system to fight their cancer, such as CAR T-cell therapy and bispecific antibodies.
These are treatments that are now being studied and are making an impact earlier in the disease course, including one now being studied as the very first treatment a patient might receive for their lymphoma.
PANCREATIC CANCER PATIENT SURVIVAL DOUBLED WITH HIGH DOSE OF COMMON VITAMIN, STUDY FINDS
These treatments are helping us to be less dependent on chemotherapies (which may be effective but have broad side effects) for the treatment of lymphomas.
Q: What are your predictions for cancer research in 2025?
A: Every year we are improving the curative treatment options we have for specific types of lymphomas, such as diffuse large B-cell lymphoma (DLBCL), which is the most common lymphoma we see.
We are also gradually becoming better able to offer these treatments closer to – or in – patients’ homes and communities, so they can receive the best care as close to home as possible.
“Every year, we are improving the curative treatment options we have for specific types of lymphomas.”
I believe that in 2025, we will continue to see more advancement in immunotherapies, development of more targeted therapies (including oral medicines), and hopefully soon the approval of next generations of immunotherapies that may work for patients who have already received today’s immunotherapies but need more treatment options.
Q: How has the state of cancer in your specialty area changed and evolved over the past decade?
A: It has changed and evolved dramatically. A decade ago, care for lymphomas was primarily chemotherapy-based. Now, we are shifting rapidly away from chemotherapies in some types of lymphomas in favor of immunotherapies and targeted oral therapies that lead to excellent long-term outcomes for patients, with fewer side effects than historical treatments.
As 2024 comes to an end, experts are looking back and reflecting on the discoveries and advances that have been made in the field of cancer treatment and prevention. (iStock)
Q: What can people do to reduce their cancer risk?
A: We think of lymphomas as diseases of aging for most patients. Some patients may have select risk factors, such as being on specific immunosuppressants or having exposure to very specific industrial chemicals.
Those risks may or may not be so modifiable for patients, and they represent the minority of patients who develop lymphoma.
AI DETECTS WOMAN’S BREAST CANCER AFTER ROUTINE SCREENING MISSED IT: ‘DEEPLY GRATEFUL’
While it is not entirely clear what modifiable risks patients may have, there is ongoing work to help better answer that question. However, we know that the better general health someone is in, the more likely they are to have any and all treatment options available to them.
I would say that for most people, exercising regularly, eating well and sleeping regularly are important.
Q: Anything else people should know?
A: There is great hope and a lot of exciting science happening to help us drive toward more cures, more effective treatments and less toxic treatments for lymphomas.
We have already made major strides in the last decade, and we continue to build on that momentum through clinical trials that provide early access to cutting-edge therapies.

Exercising regularly, eating well and getting enough sleep are all recommended activities to help prevent cancer risk, experts agreed. (iStock)
For patients, participating in clinical trials may help to close that time gap between the treatments that are broadly available today and the treatments we expect to be available years from now.
They also provide a way for patients to contribute positively to the care patients in future generations may receive, which I have been told by many of my patients is something they really want to do and something that is important to them.
Erika Hamilton, MD, director of breast cancer research

Erika Hamilton, M.D., is director of breast cancer research at Sarah Cannon Research Institute in Nashville, Tennessee. (Sarah Cannon Research Institute)
Q: What do you see as the most important cancer advances in 2024?
A: The two most exciting focuses of 2024 were 1) expansion of targeted therapies in the curative setting for hormonally driven breast cancer and 2) antibody drug conjugates.
First, three different CDK4/6 inhibitors have been approved in the metastatic setting, and they improve survival and outcomes.

Treatments that are precisely tailored to the genetic makeup of a person’s cancer are becoming more widely available, experts say. (iStock)
In 2024, we saw the approval of a second one in the curative setting, enabling us to identify the highest-risk patients and offer them something additional to endocrine therapy to improve cure rates.
Second, we now have multiple antibody drug conjugates approved across all types of breast cancer. These therapies target a chemotherapy drug directly to the tumor via an antibody-honing mechanism and largely spare normal body cells.
“I anticipate drugs that are better tolerated with decreased side effects for patients, and a continued emphasis on personalized medicine.”
Q: What are your predictions for cancer advances in 2025?
A: I anticipate seeing more targeted agents in 2025 and the approval of antibody drug conjugates in curative early breast cancer — currently, most are only approved in metastatic cancer.
[I also anticipate] drugs that are better tolerated with decreased side effects for patients, and a continued emphasis on personalized medicine.
Q: How has the state of cancer in your specialty area changed and evolved over the past decade?
A: In 2024, truly personalized medicine is possible, from mutation testing to direct targeted therapy to what a cancer needs to grow — as well as being able to provide many HR+ breast cancer patients with curative chemotherapy through personalized risk stratification assays.
“I anticipate seeing more targeted agents in 2025 and the approval of antibody drug conjugates in curative early breast cancer,” said Hamilton (not pictured). (iStock)
Q: What can people do to reduce their cancer risk?
A: Continued breast screening with mammograms yearly is really important to find cancers earlier when a cure is more likely.
People can also reduce their risk through avoiding alcohol and cigarettes and making sure they get regular exercise and maintain a normal body weight.
Vivek Subbiah, MD, chief of early-phase drug development

Vivek Subbiah, M.D., is chief of early-phase drug development at Sarah Cannon Research Institute in Nashville, Tennessee. (Sarah Cannon Research Institute )
Q: What do you see as the most important cancer advances in 2024?
A: In 2024, precision cancer treatment made big strides with many new drug approvals by the FDA, specifically for treatments guided by specific biomarkers, which means treatments can be more precisely tailored to the genetic makeup of a person’s cancer.
BREAST CANCER VACCINE UPDATE FROM CLEVELAND CLINIC: ‘A NEW ERA’
A key change was moving some therapies from faster, temporary approval processes to full approval, showing strong evidence that these targeted therapy drugs, such as tepotinib and amivantamab for certain types of lung cancer, are effective and safe.
There were also new drug approvals for rare cancers, including tovorafenib, a BRAF precision medicine for a rare type of brain tumor in children, and afamitresgene autoleucel, a type of immunotherapy for a rare cancer called synovial sarcoma. This highlights important progress in treating these challenging conditions.

“In 2024, precision cancer treatment made big strides with many new drug approvals by the FDA, specifically for treatments guided by specific biomarkers,” Subbiah (not pictured) told Fox News Digital. (iStock)
We have also seen the approval of precision therapies that work on different types of cancer — not just one specific cancer. This is what we call “tissue-agnostic therapies.”
One such drug is an antibody drug conjugate called trastuzumab deruxtecan, which acts like a smart missile targeting HER2-positive cancers. Another is repotrectinib, which works on any cancer that has the NTRK biomarker, regardless of where it is in the body.
Q: What are your predictions for cancer advances in 2025?
A: By 2025, cancer research is likely to see advancements in precision oncology and the use of artificial intelligence.
In precision oncology, we can expect more personalized treatment plans based on an individual’s genetic makeup, leading to more effective and targeted therapies with fewer side effects.
Additionally, AI will likely play a larger role in analyzing vast amounts of data to identify new drug targets, predict patient responses to treatments and enhance early detection methods.
These advancements have the potential to improve cancer diagnosis, treatment and overall patient outcomes.
Q: How has the state of cancer in your specialty area changed and evolved over the past decade?
A: In the last 10 years, cancer treatment has changed dramatically. By using genetic information to create personalized treatments that match the specific details of each person’s cancer, therapies are more effective and less harmful.
“In precision oncology, we can expect more personalized treatment plans based on an individual’s genetic makeup,” Subbiah said. (iStock)
New technologies such as analyzing cancer’s genetic profile, blood tests that detect cancer, and treatments that boost the immune system have greatly improved how we diagnose, track and treat cancer, leading to better results for patients.
Q: What can people do to reduce their cancer risk?
A: To lower the risk of cancer, people can avoid smoking, eat a healthy diet, exercise regularly, limit alcohol, protect their skin from the sun and maintain a healthy weight.
Q: Anything else people should know?
A: Get vaccines for viruses like HPV and hepatitis B, as they can lead to some cancers. Also, go for regular health checks to catch any signs of cancer early.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
We are in a unique time when treatments can be tailored specifically to each person, and many of these are available through clinical trials. If you or a loved one is diagnosed with cancer, ask your doctor if there are any clinical trials that might be a good fit.
Meredith McKean, MD, MPH, director of melanoma and skin cancer research

Meredith McKean, M.D., MPH, is the director of melanoma and skin cancer research at Sarah Cannon Research Institute in Nashville, Tennessee. (Sarah Cannon Research Institute)
Q: What do you see as the most important cancer advances in 2024?
A: The first cellular therapy, Lifileucel, was approved in melanoma after decades of research in academia and industry.
“We are in a unique time when treatments can be tailored specifically to each person, and many of these are available through clinical trials.”
This is a significant step forward for both patients with melanoma, but also the field of oncology at large.
Q: What are your predictions for cancer advances in 2025?
A: As we look to bring effective therapies from the metastatic setting into early stages of disease, we are anxiously awaiting updates in the next 18 to 24 months for a number of ongoing trials for combination therapy for patients with high-risk stage 2 or 3 melanoma.

In 2024, the first cellular therapy, Lifileucel, was approved in melanoma after decades of research, according to Meredith McKean, M.D., of the Sarah Cannon Research Institute (not pictured). (iStock)
Q: How has the state of cancer in your specialty area changed and evolved over the past decade?
A: Outcomes for melanoma have significantly changed over the past 10 years.
For more Health articles, visit www.foxnews.com/health
The five-year survival for patients with a diagnosis of stage 4 melanoma was less than 5% before 2010, and now clinical trials have shown that more than 50% of patients are still alive 10 years after being treated with FDA-approved immune checkpoint inhibitors.
Q: What can people do to reduce their cancer risk?
A: Lifelong sun protective measures, such as wearing sunscreen, avoiding direct UV exposure during peak hours of 10 a.m. to 2 p.m., and avoiding tanning beds continue to be important starting at an early age.
-

 Business1 week ago
Business1 week agoFreddie Freeman's World Series walk-off grand slam baseball sells at auction for $1.56 million
-
/cdn.vox-cdn.com/uploads/chorus_asset/file/23951353/STK043_VRG_Illo_N_Barclay_3_Meta.jpg)
/cdn.vox-cdn.com/uploads/chorus_asset/file/23951353/STK043_VRG_Illo_N_Barclay_3_Meta.jpg) Technology1 week ago
Technology1 week agoMeta’s Instagram boss: who posted something matters more in the AI age
-
/cdn.vox-cdn.com/uploads/chorus_asset/file/24924653/236780_Google_AntiTrust_Trial_Custom_Art_CVirginia__0003_1.png)
/cdn.vox-cdn.com/uploads/chorus_asset/file/24924653/236780_Google_AntiTrust_Trial_Custom_Art_CVirginia__0003_1.png) Technology4 days ago
Technology4 days agoGoogle’s counteroffer to the government trying to break it up is unbundling Android apps
-
News1 week ago
East’s wintry mix could make travel dicey. And yes, that was a tornado in Calif.
-

 Politics5 days ago
Politics5 days agoIllegal immigrant sexually abused child in the U.S. after being removed from the country five times
-

 News5 days ago
News5 days agoNovo Nordisk shares tumble as weight-loss drug trial data disappoints
-

 Entertainment5 days ago
Entertainment5 days ago'It's a little holiday gift': Inside the Weeknd's free Santa Monica show for his biggest fans
-

 Politics1 week ago
Politics1 week agoTrump taps Richard Grenell as presidential envoy for special missions, Edward S. Walsh as Ireland ambassador















