Science
Sea urchins made to order: Scripps scientists make transgenic breakthrough

Consider the sea urchin. Specifically, the painted urchin: Lytechinus pictus, a prickly Ping-Pong ball from the eastern Pacific Ocean.
The species is a smaller and shorter-spined cousin of the purple urchins devouring kelp forests. They produce massive numbers of sperm and eggs that fertilize outside of their bodies, allowing scientists to watch the process of urchin creation up close and at scale. One generation gives rise to the next in four to six months. They share more genetic material with humans than fruit flies do and can’t fly away — in short, an ideal lab animal for the developmental biologist.
Scientists have been using sea urchins to study cell development for roughly 150 years. Despite urchins’ status as super reproducers, practical concerns often compel scientists to focus their work on more easily accessible animals: mice, fruit flies, worms.
Scientists working with mice, for example, can order animals online with the specific genetic properties they are hoping to study — transgenic animals, whose genes have been artificially tinkered with to express or repress certain traits.
Researchers working with urchins typically have to spend part of their year collecting them from the ocean.
“Can you imagine if mouse researchers were setting a mousetrap every night, and whatever it is they caught is what they studied?” said Amro Hamdoun, a professor at UC San Diego’s Scripps Institution of Oceanography.
UC San Diego professor Amro Hamdoun holds a painted urchin. His breakthrough creating the creatures could lead to developments in science and medicine.
(Sandy Huffaker / For The Times)
Marine invertebrates represent about 40% of the animal world’s biological diversity yet appear in a scant fraction of a percentage of animal-based studies. What if researchers could access sea urchins as easily as mice? What if it were possible to make and raise lines of transgenic urchins?
How much more could we learn about how life works?
“You know how during the pandemic, everyone was making sourdough? I’m not good at making sourdough,” Hamdoun said recently at his office in Scripps’ Hubbs Hall. He set his sights instead on a project of a different sort: a new transgenic lab animal, “a fruit fly from the sea.”
In March, Hamdoun’s lab published a paper on the bioRxiv preprint server demonstrating the successful insertion of a piece of foreign DNA — specifically, a fluorescent protein from a jellyfish — into the genome of a painted urchin that passed the change down to its offspring.
The result is the first transgenic sea urchin, one that happens to glow like a Christmas bulb under a fluorescent light. (The paper has been submitted for peer review.)
The animals are the first transgenic echinoderms, the phylum that includes starfish, sea cucumbers and other marine animals. Hamdoun’s mission is to make genetically modified urchins available to researchers anywhere, not just those who happen to work in research facilities at the edge of the Pacific Ocean.

Postdoctoral researcher Elliot Jackson works with sea urchin eggs in a lab at Scripps.
(Sandy Huffaker / For The Times)
“If you look at some of the other model organisms, like Drosophila [fruit flies], zebrafish and mouse, there are well-established resource centers,” said Elliot Jackson, a postdoctoral researcher at Scripps and lead author of the paper. “If you want a transgenic line that labels the nervous system, you could probably get that. You could order it. And that’s what we hope we can be for sea urchins.”
Being able to genetically modify an animal supercharges what scientists can learn from it, with implications far beyond any individual species.
“It will transform sea urchins as a model for understanding neurobiology, for understanding developmental biology, for understanding toxicology,” said Christopher Lowe, a Stanford professor of biology who was not involved in the research.
The lab’s breakthrough, and its focus on making the animals freely available to fellow scientists, will “allow us to explore how evolution has solved a lot of really complicated life problems,” he said.
Researchers tend to study mice, flies and the like not because the animals’ biology is best suited to answer their questions but because “all the tools that were necessary to get at your questions were built up in just a few species,” said Deirdre Lyons, an associate professor of biology at Scripps who worked with Hamdoun on early research related to the project.
Expanding the range of animals available for sophisticated lab work is like adding colors to an artist’s palette, Lyons said: “Now you can go get the color that you really want, that best fits your vision, rather than being stuck with a few models.”

Painted urchins and humans live vastly different lives but genetically are quite similar.
(Sandy Huffaker / For The Times)
On the ground floor of Hamdoun’s office building is the Hubbs Hall experimental aquarium, a garage-like space crammed with tanks full of recirculating seawater and a motley assortment of marine life.
On a recent visit, Hamdoun reached into a tank and gently dislodged a painted urchin. It scooched with surprising speed across an outstretched palm, as if exploring alien terrain.
The last common ancestor of L. pictus and Homo sapiens lived at least 550 million years ago. Despite the different evolutionary paths we’ve since traveled, our genomes reveal a shared biological heritage.
The genetic instructions that drive the transformation of a single zygote into a living body are strikingly similar in our two species. Specialized systems differentiating from a single fertilized egg and the translation of a jumble of proteins into a singular living thing — on the cellular level, all of that proceeds in much the same way for urchins and people.
These animals are “really fundamental to our understanding of all of life,” Hamdoun said, placing the urchin back in its tank. “And historically, very inaccessible genetically.”
The experimental aquarium was built in the 1970s, when scooping life from the sea was the only way to acquire research specimens. A few floors up in Hubbs Hall, Hamdoun led the way into the urchin nursery — the first large-scale effort to raise successive generations of the animals in a laboratory. At any given moment, the team has 1,000 to 2,000 sea urchins in various stages of development.

Hamdoun points to transgenic sea urchins his lab is raising at Scripps.
(Sandy Huffaker / For The Times)
Row upon row of tiny plastic tanks stood against a wall, each containing a lentil-size juvenile urchin. A strip of tape on each tank noted the animal’s genetic modification and date of fertilization. On some, a second bit of tape indicated animals that had the modification in their sex cells’ DNA, meaning it could be passed down to offspring. (For this reason, the lab keeps its urchins scrupulously separate from the wild population.)
“One of the big questions in all of biology is to understand how the series of instructions in the genome gives you whatever phenotype you want to study,” Hamdoun said — essentially, how the string of amino acids that is an animal’s genetic code gives rise to the characteristics of the living, respiring creature. “One of the fundamental things you have to do is be able to modify that genome, and then study what the outcome is.”
He pointed to a tank containing a tiny urchin from whose genetic code the protein ABCD1 has been snipped.
ABCD1 acts like a bouncer, Hamdoun explained, parking along the cell membrane and ejecting foreign molecules. The protein’s action can preserve the cell from harmful substances but can sometimes work against an organism’s best interest, as when it prevents the cell from absorbing a necessary medication.
Researchers using urchins in which that protein no longer works can study the movement of a molecule through an organism — DDT, for example — and measure how much of the substance ends up in the cell without the confounding interference of ABCD1. They can reverse-engineer how big a role ABCD1 plays in preventing a cell from absorbing a drug.
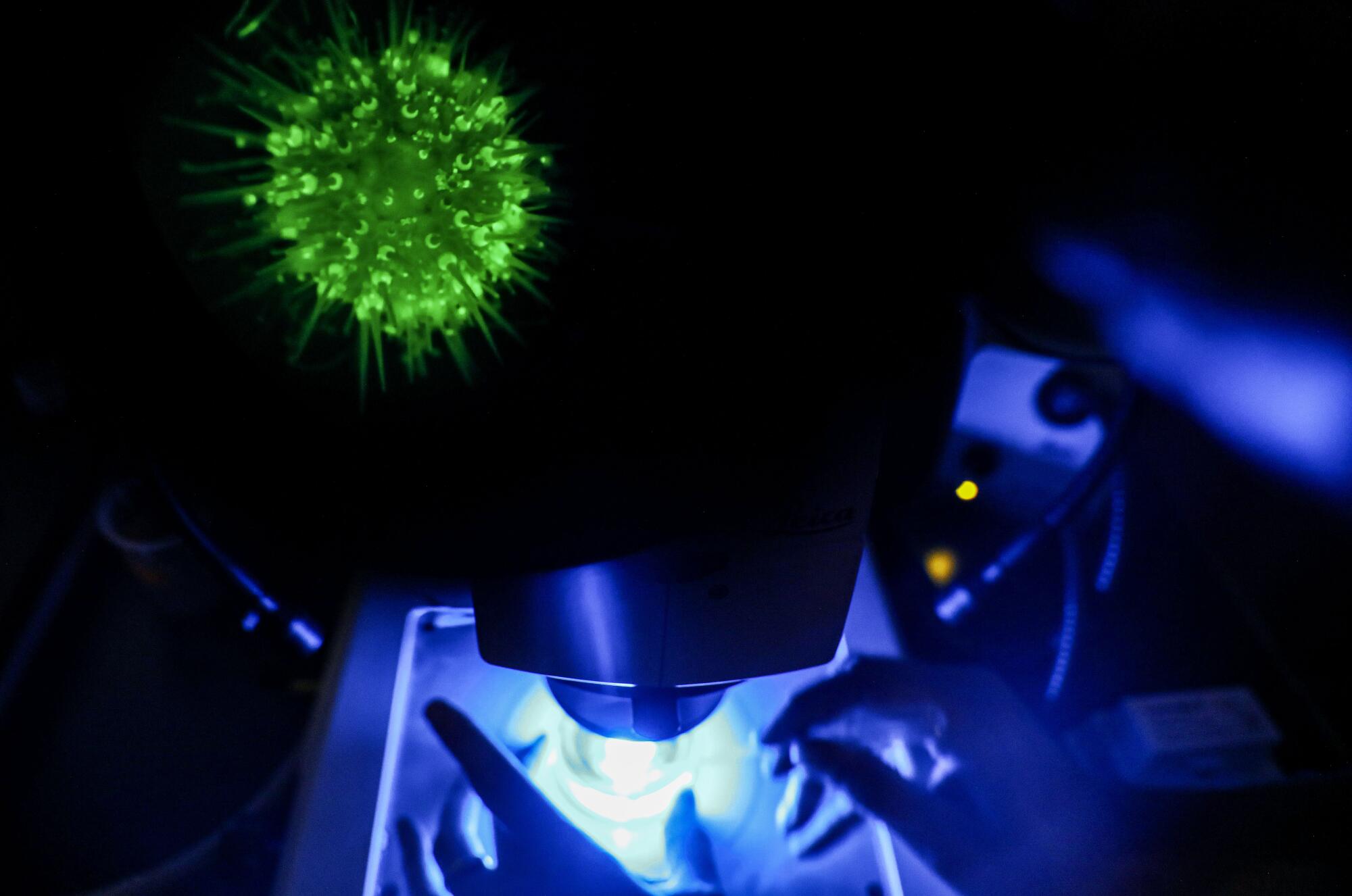
One biology professor said Scripps’ work will transform sea urchins as a model for research.
(Sandy Huffaker / For The Times)
And then there are the fluorescent urchins.
“The magic happens in this room,” Jackson said, walking into a narrow office with $1 million worth of microscopes at one end and a decades-old hand-cranked centrifuge bolted to a table at another.
He placed a petri dish containing three pencil-eraser-size transgenic urchins under a microscope. At 120 times its size, each looked like the Times Square New Year’s Eve ball come to life — a glowing, wiggling creature of pentamerous radial symmetry.
Fluorescence is not just an echinoderm party trick. Lighting up the cells makes it easier for researchers to track their movement in a developing organism. Researchers can watch as the early cells of a blastula divide and reorganize into neural or cardiac tissue. Eventually, scientists will be able to turn off individual genes and see how that affects development. It will help us understand how our own species develops, and why that development doesn’t always proceed according to plan.
The lab has “done a great job. It’s really been welcomed by the community,” said Marko Horb, senior scientist and director of the National Xenopus Resource at the University of Chicago’s Marine Biological Laboratory.
Horb runs the national clearinghouse for genetically modified species of Xenopus, a clawed frog used in lab research. Funded in part by the National Institutes of Health, the center develops lines of transgenic frogs for scientific use and distributes them to researchers.
Hamdoun envisions a similar resource center for his lab’s urchins. They’ve already started sending tiny vials of transgenic urchin sperm to interested scientists, who can grow bespoke urchins with eggs acquired from Hamdoun’s lab or another source.
Hamdoun vividly recalls the time he spent earlier in his career trying to track down random snippets of DNA necessary for his research, the disappointment and frustration of writing to professors and former postdocs only to find that the material had long been lost. He’d rather future generations of scientists spend their time on discovery.
“Biology is really interesting,” he said. “The more people can get access to it, the more we’re going to learn.”

Three transgenic sea urchins in a petri dish.
(Sandy Huffaker / For The Times)

Science
Judge blocks Trump administration move to cut $600 million in HIV funding from states

A federal judge on Thursday blocked a Trump administration order slashing $600 million in federal grant funding for HIV programs in California and three other states, finding merit in the states’ argument that the move was politically motivated by disagreements over unrelated state sanctuary policies.
U.S. District Judge Manish Shah, an Obama appointee in Illinois, found that California, Colorado, Illinois and Minnesota were likely to succeed in arguing that President Trump and other administration officials targeted the U.S. Centers for Disease Control and Prevention funding for termination “based on arbitrary, capricious, or unconstitutional rationales.”
Namely, Shah wrote that while Trump administration officials said the programs were cut for breaking with CDC priorities, other “recent statements” by officials “plausibly suggest that the reason for the direction is hostility to what the federal government calls ‘sanctuary jurisdictions’ or ‘sanctuary cities.’”
Shah found that the states had shown they would “suffer irreparable harm” from the cuts, and that the public interest would not be harmed by temporarily halting them — and as a result granted the states a temporary restraining order halting the administration’s action for 14 days while the litigation continues.
Shah wrote that while he may not have jurisdiction to block a simple grant termination, he did have jurisdiction to halt an administration directive to terminate funding based on unconstitutional grounds.
“More factual development is necessary and it may be that the only government action at issue is termination of grants for which I have no jurisdiction to review,” Shah wrote. “But as discussed, plaintiffs have made a sufficient showing that defendants issued internal guidance to terminate public-health grants for unlawful reasons; that guidance is enjoined as the parties develop a record.”
The cuts targeted a slate of programs aimed at tracking and curtailing HIV and other disease outbreaks, including one of California’s main early-warning systems for HIV outbreaks, state and local officials said. Some were oriented toward serving the LGBTQ+ community. California Atty. Gen. Rob Bonta’s office said California faced “the largest share” of the cuts.
The White House said the cuts were to programs that “promote DEI and radical gender ideology,” while federal health officials said the programs in question did not reflect the CDC’s “priorities.”
Bonta cheered Shah’s order in a statement, saying he and his fellow attorneys general who sued are “confident that the facts and the law favor a permanent block of these reckless and illegal funding cuts.”
Science
Contributor: Is there a duty to save wild animals from natural suffering?

The internet occasionally erupts in horror at disturbing images of wildlife: deer with freakish black bubbles all over their faces and bodies, sore-ridden squirrels, horn-growing rabbits.
As a society, we tend to hold romanticized notions about life in the wild. We picture these rabbits nuzzling with their babies, these squirrels munching on some nuts and these deer frolicking through sunlit meadows. Yet the trend of Frankenstein creatures afflicted with various diseases is steadily peeling back this idyllic veneer, revealing the harsher realities that underpin the natural world. And we should do something about it.
First, consider that wild animals — the many trillions of them — aren’t so different from other animals we care about — like dogs and cats — or even from us. They love. They build complex social structures. They have emotions. And most important, they too experience suffering.
Many wild animals are suffering because of us. We destroy their habitats, they’re sterilized and killed by our pollution, and sometimes we hunt them down as trophies. Suffering created by humans is especially galling.
But even in the absence of human impact, wild animals still experience a great deal of pain. They starve and thirst. They get infected by parasites and diseases. They’re ripped apart by other animals. Some of us have bought into the naturalistic fallacy that interfering with nature is wrong. But suffering is suffering wherever it occurs, and we should do something about it when we can. If we have the opportunity to rescue an injured or ill animal, why wouldn’t we? If we can alleviate a being’s suffering, shouldn’t we?
If we accept that we do have an obligation to help wild animals, where should we start? Of course, if we have an obvious opportunity to help an animal, like a bird with a broken wing, we ought to step in, maybe take it to a wildlife rescue center if there are any nearby. We can use fewer toxic products and reduce our overall waste to minimize harmful pollution, keep fresh water outside on hot summer days, reduce our carbon footprint to prevent climate-change-induced fires, build shelter for wildlife such as bats and bees, and more. Even something as simple as cleaning bird feeders can help reduce rates of disease in wild animals.
And when we do interfere in nature in ways that affect wild animals, we should do so compassionately. For example, in my hometown of Staten Island, in an effort to combat the overpopulation of deer (due to their negative impact on humans), officials deployed a mass vasectomy program, rather than culling. And it worked. Why wouldn’t we opt for a strategy that doesn’t require us to put hundreds of innocent animals to death?
But nature is indifferent to suffering, and even if we do these worthy things, trillions will still suffer because the scale of the problem is so large — literally worldwide. It’s worth looking into the high-level changes we can make to reduce animal suffering. Perhaps we can invest in the development and dissemination of cell-cultivated meat — meat made from cells rather than slaughtered animals — to reduce the amount of predation in the wild. Gene-drive technology might be able to make wildlife less likely to spread diseases such as the one afflicting the rabbits, or malaria. More research is needed to understand the world around us and our effect on it, but the most ethical thing to do is to work toward helping wild animals in a systemic way.
The Franken-animals that go viral online may have captured our attention because they look like something from hell, but their story is a reminder that the suffering of wild animals is real — and it is everywhere. These diseases are just a few of the countless causes of pain in the lives of trillions of sentient beings, many of which we could help alleviate if we chose to. Helping wild animals is not only a moral opportunity, it is a responsibility, and it starts with seeing their suffering as something we can — and must — address.
Brian Kateman is co-founder of the Reducetarian Foundation, a nonprofit organization dedicated to reducing consumption of animal products. His latest book and documentary is “Meat Me Halfway.”
Insights
L.A. Times Insights delivers AI-generated analysis on Voices content to offer all points of view. Insights does not appear on any news articles.
Viewpoint
Perspectives
The following AI-generated content is powered by Perplexity. The Los Angeles Times editorial staff does not create or edit the content.
Ideas expressed in the piece
- Wild animals experience genuine suffering comparable to that of domesticated animals and humans, including through starvation, disease, parasitism, and predation, and society romanticizes wildlife in ways that obscure these harsh realities[1][2]
- Humans have a moral obligation to address wild animal suffering wherever possible, as suffering is morally significant regardless of whether it occurs naturally or results from human action[2]
- Direct intervention in individual cases is warranted, such as rescuing injured animals or providing fresh water during heat waves, alongside broader systemic approaches like reducing pollution and carbon emissions[2]
- Humane wildlife management strategies should be prioritized over lethal approaches when addressing human-wildlife conflicts, as demonstrated by vasectomy programs that manage overpopulation without mass culling[2]
- Large-scale technological solutions, including cell-cultivated meat to reduce predation and gene-drive technology to control disease transmission, should be pursued and researched to systematically reduce wild animal suffering at scale[2]
- The naturalistic fallacy—the belief that natural processes should never be interfered with—is fundamentally flawed when weighed against the moral imperative to alleviate suffering[2]
Different views on the topic
The search results provided do not contain explicit opposing viewpoints to the author’s argument regarding a moral duty to intervene in wild animal suffering. The available sources focus primarily on the author’s work on reducing farmed animal consumption through reducetarianism and factory farming advocacy[1][3][4], rather than perspectives that directly challenge the premise that humans should work to alleviate wild animal suffering through technological or ecological intervention.
Science
Contributor: Factory farming of fish is brewing pathogens

The federal government recently released new dietary guidelines aimed at “ending the war on protein” and steering Americans toward “real foods” — those with few ingredients and no additives. Seafood plays a starring role. But the fish that health advocates envision appearing on our plates probably won’t be caught in the crystal blue waters we’d like to imagine.
Over the past few decades, the seafood industry has completely revolutionized how it feeds the world. As many wild fish populations have plummeted, hunted to oblivion by commercial fleets, fish farming has become all the rage, and captive-breeding facilities have continually expanded to satiate humanity’s ravenous appetite. Today, the aquaculture sector is a $300-billion juggernaut, accounting for nearly 60% of aquatic animal products used for direct human consumption.
Proponents of aquaculture argue that it helps feed a growing human population, reduces pressure on wild fish populations, lowers costs for consumers and creates new jobs on land. Much of that may be correct. But there is a hidden crisis brewing beneath the surface: Many aquaculture facilities are breeding grounds for pathogens. They’re also a blind spot for public health authorities.
On dry land, factory farming of cows, pigs and chickens is widely reviled, and for good reason: The unsanitary and inhumane conditions inside these facilities contribute to outbreaks of disease, including some that can leap from animals to humans. In many countries, aquaculture facilities aren’t all that different. Most are situated in marine and coastal areas, where fish can be exposed to a sinister brew of human sewage, industrial waste and agricultural runoff. Fish are kept in close quarters — imagine hundreds of adult salmon stuffed into a backyard swimming pool — and inbreeding compromises immune strength. Thus, when one fish invariably falls ill, pathogens spread far and wide throughout the brood — and potentially to people.
Right now, there are only a handful of known pathogens — mostly bacteria, rather than viruses — that can jump from aquatic species to humans. Every year, these pathogens contribute to the 260,000 illnesses in the United States from contaminated fish; fortunately, these fish-borne illnesses aren’t particularly transmissible between people. It’s far more likely that the next pandemic will come from a bat or chicken than a rainbow trout. But that doesn’t put me at ease. The ocean is a vast, poorly understood and largely unmonitored reservoir of microbial species, most of which remain unknown to science. In the last 15 years, infectious diseases — including ones that we’ve known about for decades such as Ebola and Zika — have routinely caught humanity by surprise. We shouldn’t write off the risks of marine microbes too quickly.
My most immediate concern, the one that really makes me sweat, is the emergence of drug-resistant bacteria among farmed fish. Aquaculturists are well aware that their fish often live in a festering cesspool, and so many growers will mix antibiotics — including ones that the World Health Organization considers medically important for people — into fish feed, or dump them straight into water, to avoid the consequences of crowded conditions and prevent rampant illness. It would be more appropriate to use antibiotics in animals only when they are sick.
Because of this overuse for prevention purposes, more antibiotics are used in seafood raised by aquaculture than are used in humans or for other farmed animals per kilogram. Many of these molecules will end up settling in the water or nearby sediment, where they can linger for weeks. In turn, the 1 million individual bacteria found in every drop of seawater will be put to the evolutionary test, and the most antibiotic-resistant will endure.
Numerous researchers have found that drug-resistant strains of bacteria are alarmingly common in the water surrounding aquaculture facilities. In one study, evidence of antibiotic resistance was found in over 80% of species of bacteria isolated from shrimp sold in multiple countries by multiple brands.
Many drug-resistant strains in aquatic animals won’t be capable of infecting humans, but their genes still pose a threat through a process known as horizontal transfer. Bacteria are genetic hoarders. They collect DNA from their environment and store it away in their own genome. Sometimes, they’ll participate in swap meets, trading genes with other bacteria to expand their collections. Beginning in 1991, for example, a wave of cholera infected nearly a million people across Latin America, exacerbated by a strain that may have picked up drug-resistant adaptations while circulating through shrimp farms in Ecuador.
Today, drug-resistant bacteria kill over a million people every year, more than HIV/AIDS. I’ve seen this with my own eyes as a practicing tuberculosis doctor. I am deeply fearful of a future in which the global supply of fish — a major protein source for billions of people — also becomes a source of untreatable salmonella, campylobacter and vibrio. We need safer seafood, and the solutions are already at our fingertips.
Governments need to lead by cracking down on indiscriminate antibiotic use. It is estimated that 70% of all antibiotics used globally are given to farm animals, and usage could increase by nearly 30% over the next 15 years. Regulation to promote prudent use of antibiotics in animals, however, has proven effective in Europe, and sales of veterinary antibiotics decreased by more than 50% across 25 European countries from 2011 to 2022. In the United States, the use of medically important antibiotics in food animals — including aquatic ones — is already tightly regulated. Most seafood eaten in the U.S., however, is imported and therefore beyond the reach of these rules. Indeed, antibiotic-resistance genes have already been identified in seafood imported into the United States. Addressing this threat should be an area of shared interest between traditional public health voices and the “Make America Healthy Again” movement, which has expressed serious concerns about the health effects of toxins.
Public health institutions also need to build stronger surveillance infrastructure — for both disease and antibiotic use — in potential hotspots. Surveillance is the backbone of public health, because good decision-making is impossible without good data. Unfortunately, many countries — including resource-rich countries — don’t robustly track outbreaks of antibiotic-resistant pathogens in farmed animals, nor do they share data on antibiotic use in farmed animals. By developing early warning systems for detecting antibiotic resistance in aquatic environments, rapid response efforts involving ecologists, veterinarians and epidemiologists can be mobilized as threats arise to avert public health disasters.
Meanwhile, the aquaculture industry should continue to innovate. Genetic technologies and new vaccines can help prevent rampant infections, while also improving growth efficiency that could allow for more humane conditions.
For consumers, the best way to stay healthy is simple: Seek out antibiotic-free seafood at the supermarket, and cook your fish (sorry, sushi lovers).
There’s no doubt that aquaculture is critical for feeding a hungry planet. But it must be done responsibly.
Neil M. Vora is a practicing physician and the executive director of the Preventing Pandemics at the Source Coalition.
-

 Politics1 week ago
Politics1 week agoWhite House says murder rate plummeted to lowest level since 1900 under Trump administration
-

 Alabama6 days ago
Alabama6 days agoGeneva’s Kiera Howell, 16, auditions for ‘American Idol’ season 24
-

 San Francisco, CA1 week ago
San Francisco, CA1 week agoExclusive | Super Bowl 2026: Guide to the hottest events, concerts and parties happening in San Francisco
-

 Ohio1 week ago
Ohio1 week agoOhio town launching treasure hunt for $10K worth of gold, jewelry
-

 Culture1 week ago
Culture1 week agoIs Emily Brontë’s ‘Wuthering Heights’ Actually the Greatest Love Story of All Time?
-

 News1 week ago
News1 week agoThe Long Goodbye: A California Couple Self-Deports to Mexico
-

 Politics1 week ago
Politics1 week agoTrump admin sued by New York, New Jersey over Hudson River tunnel funding freeze: ‘See you in court’
-

 Science1 week ago
Science1 week agoTuberculosis outbreak reported at Catholic high school in Bay Area. Cases statewide are climbing















