Science
New drug's potentially fatal side effects obscured by 'soothing acronym,' doctors say
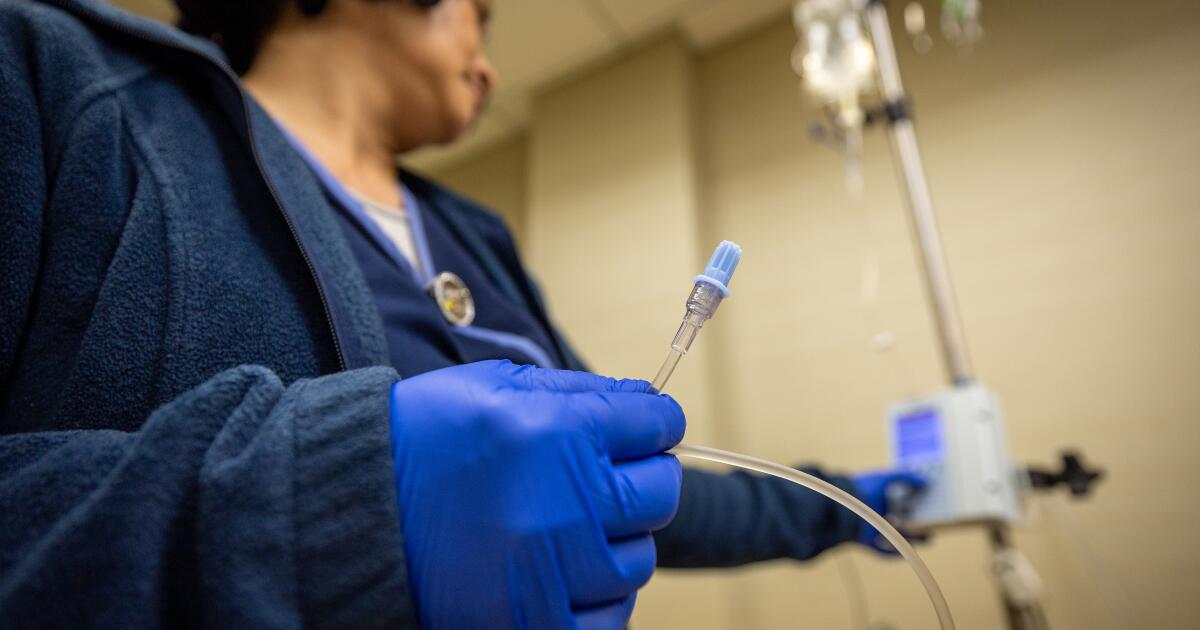
Seventy-nine-year-old Genevieve Lane volunteered to take the Alzheimer’s drug Leqembi in a clinical trial because she was forgetting words and misplacing her keys.
Infusions of the drug gave her headaches so severe they sent her to bed. A week after the third dose, she was at a restaurant with her best friend when her speech slurred and she had a seizure. Five days later she was dead.
An autopsy found that Lane died of a mysterious side effect that has a name that sounds like it might be part of an Italian opera, but has doctors on edge.
The complication called ARIA has nothing to do with music. It is a term adopted by an influential group of pharmaceutical executives and academic scientists to describe potentially fatal bleeding and swelling in the brain caused by drugs like Leqembi.
“Mom believed the drug would help slow progression of her memory problems or do nothing,” said Lane’s daughter, Yvonne Battaglia. “She didn’t know it might kill her.”
Genevieve Lane, 79, of The Villages, Fla., not long before she died in September, 2022. An autopsy blamed Leqembi, a drug for Alzheimer’s disease.
(Lane family)
Lane’s death, and that of two other trial participants, has raised concerns among some doctors, who question whether Leqembi’s risks are worth its benefits, particularly for the population of older adults it was approved for.
Some of these doctors are urging that a new name be given to the drug’s potential side effects to better alert healthcare professionals to its risks.
ARIA is short for “amyloid-related imaging abnormalities,” with imaging referring to the MRI scans needed to find the brain bleeding and swelling.
“Clearly, it is more than just an imaging abnormality,” said Dr. Matthew Schrag, a Vanderbilt University neurologist, who helped with an autopsy that concluded Lane died of brain swelling and bleeding that was likely caused by Leqembi.
“My feeling is that ARIA is too euphemistic of a term. It conveys that this isn’t serious, and it certainly can be,” he said.
Leqembi, known generically as lecanemab, is a monoclonal antibody that works to remove a protein called amyloid from the brain. It received full Food and Drug Administration approval in July.
Eisai, a Japanese drugmaker that has partnered with Biogen, is promoting Leqembi to doctors and people concerned about their memories.
“Get ahead & stay ahead for longer,” an Eisai website says about the drug. It also says that “ARIA usually occurs early in treatment and is usually asymptomatic, although serious and life-threatening events rarely can occur.”
In a recent article, a Stanford neurologist and his colleagues detailed their concerns about ARIA, which they called a “soothing acronym” for brain bleeding and swelling.
“It does certainly have the ring of something that a pharmaceutical company or public relations person would come up with,” Dr. Michael Greicius said in an interview.
Leqembi is approved for mild dementia and also a diagnosis known as mild cognitive impairment, where patients have more memory problems than others their age but can compensate and continue their daily activities. People with MCI have been found to be at greater risk for developing dementia; but in many cases, their memory problems stay the same or even improve.
The FDA has required that the company warn doctors about ARIA. The agency says the condition, which affected more than 20% of those taking the drug in a large trial, can be managed by requiring patients to get repeated MRI scans to look for bleeding and swelling.
“The FDA maintains that the benefits of Leqembi outweigh its risks when used according to the approved labeling,” said Dr. Teresa Buracchio, director of the FDA’s neuroscience office.
Libby Holman, an Eisai spokesperson, called ARIA “globally established nomenclature.”
Because of the risk of ARIA, some Los Angeles medical centers are taking extra precautions.
At Keck Medicine of USC, a neurologist is available 24/7 to take calls from families of those taking Leqembi, since a headache or sudden confusion can be a sign of ARIA, said Dr. Helena Chui, chair of the neurology department.
At UCLA and Cedars-Sinai Medical Center, warnings pop up in a patient’s electronic health record to ensure that all medical staff know the patient is taking Leqembi, because it can interact with certain other medications to make brain bleeding far worse.
And at all three medical centers it takes more than a single doctor to prescribe the drug. Each patient’s case must be reviewed by a panel of doctors and other staff — similar to how complex cancer cases are evaluated.
“We want to keep safety first,” said Dr. Keith Vossel, UCLA professor of neurology. “This is the most complicated, complex drug that we’ve prescribed in the dementia field.”
Other physicians say they won’t prescribe the drug.
“If there was a medication that worked, I would be the first person to use it,” said Dr. Clifford Sigel, a neurologist in Santa Monica. “But I won’t be using this in my practice.”
He pointed to a large clinical trial of Leqembi that led to its approval. It found that patients who took the drug saw their memory decline 27% more slowly — or less than half a point on an 18-point cognitive scale — than their counterparts who took a placebo. Sigel and other doctors doubt patients or their families would notice the difference.
Eisai’s Holman disputed claims that the drug does not work. She noted that a panel of outside experts convened by the FDA had voted unanimously that trial data confirmed its clinical benefit.
The name ARIA traces to July 2010 when “turmoil ensued” at an international scientific conference that the Alzheimer’s Assn. holds each year, according to an article by two scientists working in the field.
The FDA had proposed that companies testing new anti-amyloid drugs exclude any volunteer from clinical trials who had more than two brain microbleeds, according to an Alzheimer’s Assn. report. The tiny hemorrhages are sometimes found in healthy people and those with Alzheimer’s or other illnesses.
The agency also said it would require any volunteer who experienced a brain microbleed during the clinical trial to cease taking the drug.
The companies and academics working on the trials viewed the new FDA requirements as “excessively restrictive,” said the report by the association, a nonprofit that has become a powerful force in dementia science.
The industry and academic scientists feared the FDA proposal would stall research on the experimental drugs, the report said, and limit their use.
The drug companies asked the association to debate the FDA’s guidance at its Research Roundtable. Pharmaceutical and medical testing companies can become members of the roundtable by paying the association a $50,000 annual fee.
The association said that “one key question” taken up by the roundtable was whether ARIA was a temporary symptom of the new drug — much the way nausea and hair loss are side effects of chemotherapy — or evidence that anti-amyloid medicines may have more serious adverse effects. That question was never settled.
“Current knowledge doesn’t provide definitive answers to this critical question,” the association said in the 2011 report explaining the roundtable’s work.
Despite the unknowns, the roundtable proposed that volunteers be allowed into the trials even if they had as many as four brain microbleeds. The group said volunteers could keep getting the drug infusions if they developed brain bleeding as long as they did not have significant worsening of symptoms such as headaches and confusion.
The roundtable also proposed calling the brain bleeding and swelling ARIA.
The FDA “subsequently revised and updated the original advice…in a manner consistent” with the roundtable’s suggestions, wrote three of its members.
“Scientific evidence at the time led the workgroup to propose excluding people who have four or more microbleeds from clinical trials,” the association told The Times in a statement. “The FDA agreed.”
The association declined to answer questions on whether the name ARIA should be changed.
An FDA official told The Times that the industry group’s advice was just one of the factors the agency considered before it revised its 2010 guidelines.
More than a decade later, little more is known about why ARIA occurs or how to recognize it.
One problem is that a patient with ARIA can look like they’re having a stroke. And when stroke patients are taken to an emergency room, the first treatment doctors often consider is a clot-dissolving medicine called tPA, which can make brain bleeding worse.
That’s what happened to a 65-year-old woman taking Leqembi in a trial who arrived at a Chicago ER with stroke-like symptoms, according to a report published in February 2023. Doctors gave her tPA.
“As soon as they put it in her, it was like her body was on fire,” the woman’s husband said in a news story in the journal Science. “She was screaming, and it took like eight people to hold her down.”
The woman died, and an autopsy showed extensive bleeding in her brain, leading doctors to conclude the combination of the two drugs may have caused her death.
Knowing about that risk, Southern California doctors have been teaching emergency room staff to find out if patients thought to be suffering a stroke may be taking Leqembi.
“We’ve had to train and discuss this with the ER, the neuroradiology team and urgent care,” said Dr. Sarah Kremen, who leads Cedars Sinai’s Alzheimer’s clinical trial program. “You must ask this person, ‘Are you taking this medication?’”
An FDA database that collects reports of adverse drug reactions from doctors and others shows 23 deaths of patients taking Leqembi.
Holman at Eisai said it would be incorrect to assume the deaths were caused by Leqembi. She noted that Alzheimer’s patients have a higher risk of death because of the natural course of the disease.
In the large trial, less than 1% of patients died — the same rate whether they were taking the drug or the placebo.
Buracchio at the FDA said the agency takes “all adverse event reports seriously.” But she said the agency’s evaluation of the reports “must take the treated population into account,” which in this case is typically older or elderly adults.
To teach doctors about ARIA, Eisai created a website called understandingaria.com. It tells doctors that ARIA “usually resolves without intervention or treatment modification.”
In a brochure for healthcare providers, Eisai assures physicians that infusions may continue if an MRI turns up evidence of microbleeds as long as there are four or fewer and that the discomfort doesn’t disrupt the patient’s activities.
For Genevieve Lane, an MRI discovered four brain microbleeds before she started taking Leqembi in the trial.
After Lane died, an autopsy found more than 30 microbleeds in her brain, including some that could not be seen on the MRI, according to a report in Nature Communications.
The report’s authors, who included Schrag at Vanderbilt, questioned whether the pre-treatment limit of four brain microbleeds was stringent enough and called for higher standards.
The FDA told The Times that the agency had reviewed the available data and had not identified a specific number of preexisting microhemorrhages that would make it unsafe for patients to take anti-amyloid drugs like Leqembi.
“However, we will continue to monitor the accruing safety data,” the agency said.
Other doctors have questioned what happens to the memories of those who suffer ARIA, even if the bleeding and swelling appears to resolve.
Dr. Madhav Thambisetty, a senior researcher at the National Institute of Aging, said he was concerned by a report in a French medical journal about two women with mild dementia who experienced serious ARIA during a trial. One suffered severe seizures; 11 months later, her memory score dropped by nine points on a 30-point scale. The other patient developed a brain bleed described as “massive”; she lost a significant part of her vision, and her memory score declined by 12 points on the same scale.
An FDA scientist reviewing reports of patients who suffered high numbers of microbleeds in the clinical trial also noted the possible harm to their cognition in her January 2023 report on Leqembi.
One of the patients that Dr. Deniz Erten-Lyons pointed to was a 68-year-old man who had four microbleeds before starting the infusions. After treatment, he began to lose his vision and was hospitalized because of a seizure. An MRI found 96 microbleeds.
Thambisetty said he and Dr. Rob Howard of University College London wrote to Eisai last year to request information about what happened to the cognition of those who suffered ARIA in trials.
Eisai has not responded to their request, he said.
“I’m concerned about the lack of full and transparent reporting,” Thambisetty said. “It’s really important to know what happens to these patients.”
Holman said the company’s analysis of trial data showed that ARIA did not impact cognition.
“Eisai is transparent,” she said. The company follows guidelines for sharing clinical data established by PhRMA, the industry trade association, Holman said.
Greicius, the Stanford professor, also asked Eisai for trial data that would break down results for each volunteer to better understand ARIA and whether patients benefited as more amyloid was removed from their brains.
The response from Eisai, he said, was, “Thanks for your interest, but we can’t release the data.”

Science
Diablo Canyon clears last California permit hurdle to keep running

Central Coast Water authorities approved waste discharge permits for Diablo Canyon nuclear plant Thursday, making it nearly certain it will remain running through 2030, and potentially through 2045.
The Pacific Gas & Electric-owned plant was originally supposed to shut down in 2025, but lawmakers extended that deadline by five years in 2022, fearing power shortages if a plant that provides about 9 percent the state’s electricity were to shut off.
In December, Diablo Canyon received a key permit from the California Coastal Commission through an agreement that involved PG&E giving up about 12,000 acres of nearby land for conservation in exchange for the loss of marine life caused by the plant’s operations.
Today’s 6-0 vote by the Central Coast Regional Water Board approved PG&E’s plans to limit discharges of pollutants into the water and continue to run its “once-through cooling system.” The cooling technology flushes ocean water through the plant to absorb heat and discharges it, killing what the Coastal Commission estimated to be two billion fish each year.
The board also granted the plant a certification under the Clean Water Act, the last state regulatory hurdle the facility needed to clear before the federal Nuclear Regulatory Commission (NRC) is allowed to renew its permit through 2045.
The new regional water board permit made several changes since the last one was issued in 1990. One was a first-time limit on the chemical tributyltin-10, a toxic, internationally-banned compound added to paint to prevent organisms from growing on ship hulls.
Additional changes stemmed from a 2025 Supreme Court ruling that said if pollutant permits like this one impose specific water quality requirements, they must also specify how to meet them.
The plant’s biggest water quality impact is the heated water it discharges into the ocean, and that part of the permit remains unchanged. Radioactive waste from the plant is regulated not by the state but by the NRC.
California state law only allows the plant to remain open to 2030, but some lawmakers and regulators have already expressed interest in another extension given growing electricity demand and the plant’s role in providing carbon-free power to the grid.
Some board members raised concerns about granting a certification that would allow the NRC to reauthorize the plant’s permits through 2045.
“There’s every reason to think the California entities responsible for making the decision about continuing operation, namely the California [Independent System Operator] and the Energy Commission, all of them are sort of leaning toward continuing to operate this facility,” said boardmember Dominic Roques. “I’d like us to be consistent with state law at least, and imply that we are consistent with ending operation at five years.”
Other board members noted that regulators could revisit the permits in five years or sooner if state and federal laws changes, and the board ultimately approved the permit.
Science
Deadly bird flu found in California elephant seals for the first time

The H5N1 bird flu virus that devastated South American elephant seal populations has been confirmed in seals at California’s Año Nuevo State Park, researchers from UC Davis and UC Santa Cruz announced Wednesday.
The virus has ravaged wild, commercial and domestic animals across the globe and was found last week in seven weaned pups. The confirmation came from the U.S. Department of Agriculture’s National Veterinary Services Laboratory in Ames, Iowa.
“This is exceptionally rapid detection of an outbreak in free-ranging marine mammals,” said Professor Christine Johnson, director of the Institute for Pandemic Insights at UC Davis’ Weill School of Veterinary Medicine. “We have most likely identified the very first cases here because of coordinated teams that have been on high alert with active surveillance for this disease for some time.”
Since last week, when researchers began noticing neurological and respoiratory signs of the disease in some animals, 30 seals have died, said Roxanne Beltran, a professor of ecology and evolutionary biology at UC Santa Cruz. Twenty-nine were weaned pups and the other was an adult male. The team has so far confirmed the virus in only seven of the dead pups.
Infected animals often have tremors convulsions, seizures and muscle weakness, Johnson said.
Beltran said teams from UC Santa Cruz, UC Davis and California State Parks monitor the animals 260 days of the year, “including every day from December 15 to March 1” when the animals typically come ashore to breed, give birth and nurse.
The concerning behavior and deaths were first noticed Feb. 19.
“This is one of the most well-studied elephant seal colonies on the planet,” she said. “We know the seals so well that it’s very obvious to us when something is abnormal. And so my team was out that morning and we observed abnormal behaviors in seals and increased mortality that we had not seen the day before in those exact same locations. So we were very confident that we caught the beginning of this outbreak.”
In late 2022, the virus decimated southern elephant seal populations in South America and several sub-Antarctic Islands. At some colonies in Argentina, 97% of pups died, while on South Georgia Island, researchers reported a 47% decline in breeding females between 2022 and 2024. Researchers believe tens of thousands of animals died.
More than 30,000 sea lions in Peru and Chile died between 2022 and 2024. In Argentina, roughly 1,300 sea lions and fur seals perished.
At the time, researchers were not sure why northern Pacific populations were not infected, but suspected previous or milder strains of the virus conferred some immunity.
The virus is better known in the U.S. for sweeping through the nation’s dairy herds, where it infected dozens of dairy workers, millions of cows and thousands of wild, feral and domestic mammals. It’s also been found in wild birds and killed millions of commercial chickens, geese and ducks.
Two Americans have died from the virus since 2024, and 71 have been infected. The vast majority were dairy or commercial poultry workers. One death was that of a Louisiana man who had underlying conditions and was believed to have been exposed via backyard poultry or wild birds.
Scientists at UC Santa Cruz and UC Davis increased their surveillance of the elephant seals in Año Nuevo in recent years. The catastrophic effect of the disease prompted worry that it would spread to California elephant seals, said Beltran, whose lab leads UC Santa Cruz’s northern elephant seal research program at Año Nuevo.
Johnson, the UC Davis researcher, said the team has been working with stranding networks across the Pacific region for several years — sampling the tissue of birds, elephant seals and other marine mammals. They have not seen the virus in other California marine mammals. Two previous outbreaks of bird flu in U.S. marine mammals occurred in Maine in 2022 and Washington in 2023, affecting gray and harbor seals.
The virus in the animals has not yet been fully sequenced, so it’s unclear how the animals were exposed.
“We think the transmission is actually from dead and dying sea birds” living among the sea lions, Johnson said. “But we’ll certainly be investigating if there’s any mammal-to-mammal transmission.”
Genetic sequencing from southern elephant seal populations in Argentina suggested that version of the virus had acquired mutations that allowed it to pass between mammals.
The H5N1 virus was first detected in geese in China in 1996. Since then it has spread across the globe, reaching North America in 2021. The only continent where it has not been detected is Oceania.
Año Nuevo State Park, just north of Santa Cruz, is home to a colony of some 5,000 elephant seals during the winter breeding season. About 1,350 seals were on the beach when the outbreak began. Other large California colonies are located at Piedras Blancas and Point Reyes National Sea Shore. Most of those animals — roughly 900 — are weaned pups.
It’s “important to keep this in context. So far, avian influenza has affected only a small proportion of the weaned at this time, and there are still thousands of apparently healthy animals in the population,” Beltran said in a press conference.
Public access to the park has been closed and guided elephant seal tours canceled.
Health and wildlife officials urge beachgoers to keep a safe distance from wildlife and keep dogs leashed because the virus is contagious.
Science
When slowing down can save a life: Training L.A. law enforcement to understand autism

Kate Movius moved among a roomful of Los Angeles County sheriff’s deputies, passing out a pop trivia quiz and paper prism glasses.
She told them to put on the vision-distorting glasses, and to write with their nondominant hand. As they filled out the tests, Movius moved about the City of Industry classroom pounding abruptly on tables. Then came the cowbell. An aide flashed the overhead lights on and off at random. The goal was to help the deputies understand the feeling of sensory overwhelm, which many autistic people experience when incoming stimulation exceeds their capacity to process.
“So what can you do to assist somebody, or de-escalate somebody, or get information from someone who suffers from a sensory disorder?” Movius asked the rattled crowd afterward. “We can minimize sensory input. … That might be the difference between them being able to stay calm and them taking off.”
Movius, founder of the consultancy Autism Interaction Solutions, is one of a growing number of people around the U.S. working to teach law enforcement agencies to recognize autistic behaviors and ensure that encounters between neurodevelopmentally disabled people and law enforcement end safely.
She and City of Industry Mayor Cory Moss later passed out bags filled with tools donated by the city to aid interactions: a pair of noise-damping headphones to decrease auditory input, a whiteboard, a set of communication cards with words and images to point to, fidget toys to calm and distract.
“The thing about autistic behavior when it comes to law enforcement is a lot of it may look suspicious, and a lot of it may feel very disrespectful,” said Movius, who is also the parent of an autistic 25-year-old man. Responding officers, she said, “are not coming in thinking, ‘Could this be a developmentally disabled person?’ I would love for them to have that in the back of their minds.”
A sheriff’s deputy reads a pamphlet on autism during the training program.
(Genaro Molina / Los Angeles Times)
Autism spectrum disorder is a developmental condition that manifests differently in nearly every person who has it. Symptoms cluster around difficulties in communication, social interaction and sensory processing.
An autistic person stopped by police might hold the officer’s gaze intensely or not look at them at all. They may repeat a phrase from a movie, repeat the officer’s question or temporarily lose their ability to speak. They might flee.
All are common involuntary responses for an autistic person in a stressful situation, which a sudden encounter with law enforcement almost invariably is. To someone unfamiliar with the condition, all could be mistaken for intoxication, defiance or guilt.
Autism rates in the U.S. have increased nearly fivefold since the Centers for Disease Control began tracking diagnoses in 2000, a rise experts attribute to broadening diagnostic criteria and better efforts to identify children who have the condition.
The CDC now estimates that 1 in 31 U.S. 8-year-olds is autistic. In California, the rate is closer to 1 in 22 children.
As diverse as the autistic population is, people across the spectrum are more likely to be stopped by law enforcement than neurotypical peers.
About 15% of all people in the U.S. ages 18 to 24 have been stopped by police at some point in their lives, according to federal data. While the government doesn’t track encounters for disabled people specifically, a separate study found that 20% of autistic people ages 21 to 25 have been stopped, often after a report or officer observation of a person behaving unusually.
Some of these encounters have ended in tragedy.
In 2021, Los Angeles County sheriff’s deputies shot and permanently paralyzed a deaf autistic man after family members called 911 for help getting him to a hospital.
Isaias Cervantes, 25, had become distressed about a shopping trip and started pushing his mother, his family’s attorney said at the time. He resisted as two deputies attempted to handcuff him and one of the deputies shot him, according to a county report.
In 2024, Ryan Gainer’s family called 911 for support when the 15-year-old became agitated. Responding San Bernardino County sheriff‘s deputies shot and killed him outside his Apple Valley home.
Last year, police in Pocatello, Idaho, shot Victor Perez, 17, through a chain-link fence after the nonspeaking teenager did not heed their shouted commands. He died from his injuries in April.

Sheriff’s deputies take a trivia quiz using their non-writing hands, while wearing vision-distorting glasses, as Kate Movius, standing left, and Industry Mayor Cory Moss, right, ring cowbells. The idea was to help them understand the sensory overwhelm some autistic people experience.
(Genaro Molina / Los Angeles Times)
As early as 2001, the FBI published a bulletin on police officers’ need to adjust their approach when interacting with autistic people.
“Officers should not interpret an autistic individual’s failure to respond to orders or questions as a lack of cooperation or as a reason for increased force,” the bulletin stated. “They also need to recognize that individuals with autism often confess to crimes that they did not commit or may respond to the last choice in a sequence presented in a question.”
But a review of multiple studies last year by Chapman University researchers found that while up to 60% of officers have been on a call involving an autistic person, only 5% to 40% had received any training on autism.
In response, universities, nonprofits and private consultants across the U.S. have developed curricula for law enforcement on how to recognize autistic behaviors and adapt accordingly.
The primary goal, Movius told deputies at November’s training session, is to slow interactions down to the greatest extent possible. Many autistic people require additional time to process auditory input and verbal responses, particularly in unfamiliar circumstances.
If at all possible, Movius said, wait 20 seconds for a response after asking a question. It may feel unnaturally long, she acknowledged. But every additional question or instruction fired in that time — what’s your name? Did you hear me? Look at me. What’s your name? — just decreases the likelihood that a person struggling to process will be able to respond at all.
Moss’ son, Brayden, then 17, was one of several teenagers and young adults with autism who spoke or wrote statements to be read to the deputies. The diversity of their speech patterns and physical mannerisms showed the breadth of the spectrum. Some were fluently verbal, while others communicated through signs and notes.
“This population is so diverse. It is so complicated. But if there’s anything that we can show [deputies] in here that will make them stop and think, ‘Hey, what if this is autism?’ … it is saving lives,” Moss said.

Mayor Cory Moss, left, and Kate Movius hug at the end of the training program last November. Movius started Autism Interaction Solutions after her son was born with profound autism.
(Genaro Molina / Los Angeles Times)
Some disability advocates cautioned that it takes more than isolated training sessions to ensure encounters end safely.
Judy Mark, co-founder and president of the nonprofit Disability Voices United, says she trained thousands of officers on safe autism interactions but stopped after Cervantes’ shooting. She now urges families concerned about an autistic child’s safety to call an ambulance rather than law enforcement.
“I have significant concern about these training sessions,” Mark said. “People get comfort from it, and the Sheriff’s Department can check the box.”
While not a panacea, supporters argue that a brief course is better than no preparation at all. Some years ago, Movius received a letter from a man whose profoundly autistic son slipped away as the family loaded their car at the beach. He opened the unlocked door of a police vehicle, climbed into the back and began to flail in distress.
Though surprised, the officer seated at the wheel de-escalated the situation and helped the young man find his family, the father wrote to Movius. He had just been to her training.
-

 World2 days ago
World2 days agoExclusive: DeepSeek withholds latest AI model from US chipmakers including Nvidia, sources say
-

 Massachusetts2 days ago
Massachusetts2 days agoMother and daughter injured in Taunton house explosion
-

 Montana1 week ago
Montana1 week ago2026 MHSA Montana Wrestling State Championship Brackets And Results – FloWrestling
-

 Oklahoma1 week ago
Oklahoma1 week agoWildfires rage in Oklahoma as thousands urged to evacuate a small city
-

 Louisiana5 days ago
Louisiana5 days agoWildfire near Gum Swamp Road in Livingston Parish now under control; more than 200 acres burned
-

 Denver, CO2 days ago
Denver, CO2 days ago10 acres charred, 5 injured in Thornton grass fire, evacuation orders lifted
-

 Technology6 days ago
Technology6 days agoYouTube TV billing scam emails are hitting inboxes
-

 Technology6 days ago
Technology6 days agoStellantis is in a crisis of its own making




















