Health
US man who got first pig heart transplant dies 2 months later
NEWNow you can hearken to Fox Information articles!
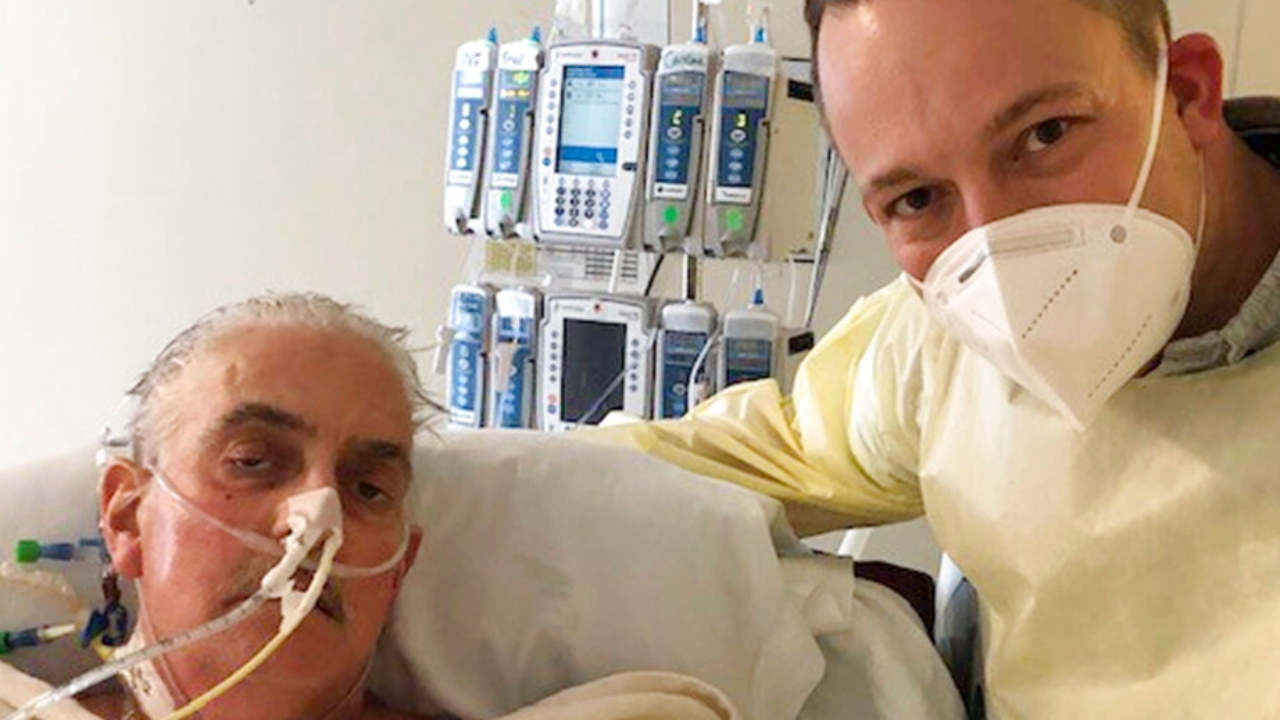
The primary particular person to obtain a coronary heart transplant from a pig has died, two months after the groundbreaking experiment, the Maryland hospital that carried out the surgical procedure introduced Wednesday.
David Bennett, 57, died Tuesday on the College of Maryland Medical Heart. Medical doctors didn’t give a precise explanation for loss of life, saying solely that his situation had begun deteriorating a number of days earlier.
MAN GETS GENETICALLY ALTERED PIG’S HEAT TRANSPLANT IN FIRST-OF-ITS-KIND PROCEDURE
Bennett’s son praised the hospital for providing the last-ditch experiment, saying the household hoped it could assist additional efforts to finish the organ scarcity.
“We’re grateful for each revolutionary second, each loopy dream, each sleepless evening that went into this historic effort,” David Bennett Jr. stated in a press release launched by the College of Maryland College of Drugs. “We hope this story may be the start of hope and never the tip.”
Medical doctors for many years have sought to at some point use animal organs for life-saving transplants. Bennett, a handyman from Hagerstown, Maryland, was a candidate for this latest try solely as a result of he in any other case confronted sure loss of life — ineligible for a human coronary heart transplant, bedridden and on life help, and out of different choices.
FILE – On this photograph supplied by the College of Maryland College of Drugs, members of the surgical workforce present the pig coronary heart for transplant into affected person David Bennett in Baltimore on Friday, Jan. 7, 2022. Bennett, the primary particular person to obtain a coronary heart transplant from a pig died Tuesday, March 8, on the College of Maryland Medical Heart, two months after the groundbreaking experiment. His loss of life was introduced Wednesday.
(Mark Teske/College of Maryland College of Drugs through AP)
After the Jan. 7 operation, Bennett’s son advised The Related Press his father knew there was no assure it could work.
Prior makes an attempt at such transplants — or xenotransplantation — have failed largely as a result of sufferers’ our bodies quickly rejected the animal organ. This time, the Maryland surgeons used a coronary heart from a gene-edited pig: Scientists had modified the animal to take away pig genes that set off the hyper-fast rejection and add human genes to assist the physique settle for the organ.
At first the pig coronary heart was functioning, and the Maryland hospital issued periodic updates that Bennett gave the impression to be slowly recovering. Final month, the hospital launched video of him watching the Tremendous Bowl from his hospital mattress whereas working together with his bodily therapist.
Bennett survived considerably longer with the gene-edited pig coronary heart than one of many final milestones in xenotransplantation — when Child Fae, a dying California toddler, lived 21 days with a baboon’s coronary heart in 1984.
“We’re devastated by the lack of Mr. Bennett. He proved to be a courageous and noble affected person who fought all the best way to the tip,” Dr. Bartley Griffith, who carried out the surgical procedure on the Baltimore hospital, stated in a press release.
PIG KIDNEY TRANSPLANT SUCCESSFULLY TESTED ON DECEASED WOMAN
The necessity for one more supply of organs is big. Greater than 41,000 transplants have been carried out within the U.S. final yr, a file — together with about 3,800 coronary heart transplants. However greater than 106,000 folks stay on the nationwide ready listing, hundreds die yearly earlier than getting an organ and hundreds extra by no means even get added to the listing, thought of an excessive amount of of a protracted shot.
The Meals and Drug Administration had allowed the dramatic Maryland experiment underneath “compassionate use” guidelines for emergency conditions. Bennett’s medical doctors stated he had coronary heart failure and an irregular heartbeat, plus a historical past of not complying with medical directions. He was deemed ineligible for a human coronary heart transplant that requires strict use of immune-suppressing medicines, or the remaining various, an implanted coronary heart pump.
Medical doctors didn’t reveal the precise explanation for Bennett’s loss of life. Rejection, an infection and different issues are dangers for transplant recipients.
However from Bennett’s expertise, “now we have gained invaluable insights studying that the genetically modified pig coronary heart can operate nicely inside the human physique whereas the immune system is sufficiently suppressed,” stated Dr. Muhammad Mohiuddin, scientific director of the Maryland college’s animal-to-human transplant program.
One subsequent query is whether or not scientists have realized sufficient from Bennett’s expertise and another current experiments with gene-edited pig organs to steer the FDA to permit a medical trial — presumably with an organ akin to a kidney that isn’t instantly deadly if it fails.
Twice final fall, surgeons at New York College received permission from the households of deceased people to briefly connect a gene-edited pig kidney to blood vessels outdoors the physique and watch them work earlier than ending life help. And surgeons on the College of Alabama at Birmingham went a step additional, transplanting a pair of gene-edited pig kidneys right into a brain-dead man in a step-by-step rehearsal for an operation they hope to attempt in dwelling sufferers presumably later this yr.
Pigs have lengthy been utilized in human medication, together with pig pores and skin grafts and implantation of pig coronary heart valves. However transplanting whole organs is rather more complicated than utilizing extremely processed tissue. The gene-edited pigs utilized in these experiments have been supplied by Revivicor, a subsidiary of United Therapeutics, certainly one of a number of biotech corporations within the working to develop appropriate pig organs for potential human transplant.

Health
Costco-brand cold and flu medication recalled by FDA: 'Not effective'

A Costco-brand cold and flu medicine has been pulled from shelves by the thousands.
A total of 8,640 boxes of Kirkland Severe Cold & Flu Plus Congestion Day and Night packs were recalled by the Food & Drug Administration (FDA) last week.
This is following the FDA’s proposal to ban the use of oral phenylephrine as an over-the-counter nasal decongestant in early November.
FOOD AND DRINK PRODUCT RECALLS IN DECEMBER: IT’S ALL ABOUT ‘PROTECTING CONSUMERS’
After “extensive review,” the FDA concluded that the active ingredient in the product (oral phenylephrine) is “not effective” as a nasal decongestant.
Nearly 9,000 boxes of Kirkland Severe Cold & Flu Plus Congestion Day and Night were recalled for quality control issues. (iStock)
Dr. Marc Siegel, Fox News senior medical analyst, said he agrees with the recent pushback against the ingredient.
“This chemical is shown to be ineffective against cold and flu in its oral form, except at a dose that has some heart toxicity and can lead to palpitations, arrhythmia and high blood pressure.”
DR. NICOLE SAPHIER’S 5 FOODS TO BOOST THE IMMUNE SYSTEM THIS COLD AND FLU SEASON
The FDA stated that the Kirkland recall was related to quality control issues, noting in the official recall that the “released product should have been rejected.”
This was a Class II recall, which the FDA describes as a “situation in which use of, or exposure to, a violative product may cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote.”

Recalled boxes of Kirkland Severe Cold & Flu Plus Congestion had lot numbers P139953 or P139815 with an August 2026 expiration date. (eBay)
Katy Dubinsky, a New York pharmacist and founder and CEO of Vitalize, confirmed with Fox News Digital that the recall most likely occurred due to deviations from CGMP (Current Good Manufacturing Practice).
“[That means] the product failed to meet required quality control standards and should have been rejected before release,” she said.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
“To guarantee all products meet strict safety and quality guidelines, the FDA made a Class II recall, which suggests low consumer risk and typically addresses issues unlikely to cause serious harm.”
For more Health articles, visit foxnews.com/health
Manufacturing inconsistencies and labeling errors can cause “many” CGMP deviations, according to the pharmacist, as well as other procedural issues rather than ingredient problems.
“Acetaminophen, dextromethorphan, guaifenesin and phenylephrine – the listed active ingredients – are widely used as well as considered safe when taken as directed,” she said.

Pharmacist Katy Dubinsky told consumers to stop using recalled lots of this product. (iStock)
Dubinsky instructed consumers to stop using the recalled lots and consult with a health care professional if concerns or symptoms arise after taking the product.
“However, there is no need for panic,” she said. “Recalls like this are important to keep products safe and help people trust the over-the-counter medications they rely on.”
Recalled boxes of the Kirkland Cold & Flu product had lot numbers P139953 or P139815 with an August 2026 expiration date.
Fox News Digital reached out to the FDA and Costco for comment.
Health
Bird flu leads to severe human illness and state of emergency; experts discuss risk
Bird flu (H5N1) continues to spark warnings around the country.
On Friday, the Centers for Disease Control and Prevention (CDC) confirmed the country’s first severe case of bird flu in a human.
The patient, who lives in southwestern Louisiana, is currently hospitalized, according to a release from the Louisiana Department of Health (LDH).
BIRD FLU SURGES IN SEVERAL US STATES WITH REPORTS OF NEW OUTBREAKS: ‘GETTING WORSE’
The infected person is known to have been exposed to sick and dead birds that are “suspected to have been infected,” the same source stated.
The LDH is working with the CDC on genomic testing of the virus infecting the hospitalized patient. Fox News Digital reached out to the department for comment.
The Centers for Disease Control and Prevention on Friday confirmed the country’s first severe case of bird flu in a human. (iStock)
There have been a total of 61 human cases throughout the country since April.
No human-to-human transmission has been reported, leading the CDC to maintain its stance that risk to the public is low.
GOVERNOR NEWSOM DECLARES STATE OF EMERGENCY IN CALIFORNIA DUE TO BIRD FLU
Despite the low risk, California Gov. Gavin Newsom on Wednesday declared a state of emergency due to the bird flu.
The declaration follows an outbreak of the virus among dairy cows in Southern California farms, according to the news release on the governor’s website.
“This proclamation is a targeted action to ensure government agencies have the resources and flexibility they need to respond quickly to this outbreak,” Newsom said in a statement.

California Gov. Gavin Newsom on Wednesday declared a state of emergency due to the bird flu. (Mario Tama)
“Building on California’s testing and monitoring system — the largest in the nation — we are committed to further protecting public health, supporting our agricultural industry, and ensuring that Californians have access to accurate, up-to-date information,” he continued.
“While the risk to the public remains low, we will continue to take all necessary steps to prevent the spread of this virus.”
Doctors discuss bird flu risk
Sam Scarpino, PhD, director of AI and life sciences at Northeastern University in Boston, said the “tragic case” in Louisiana is evidence of the “widespread nature” of H5N1 in the U.S.
“It also reinforces the very serious situation we are facing,” he told Fox News Digital.
“We need to take more decisive action to control the spread of H5N1 in animal populations.”
“We need to take more decisive action to control the spread of H5N1 in animal populations. Until then, we will continue to see human spillover cases, and some of them will unfortunately be severe.”
Dr. Marc Siegel, clinical professor of medicine at NYU Langone Health and Fox News senior medical analyst, pointed out that this severe case represents just one instance of the infection and is not necessarily cause for alarm.

No human-to-human transmission has been reported, leading the CDC to maintain its stance that risk to the public is low. (iStock)
“We’d have to know more about the patient’s particular medical details,” he told Fox News Digital. “We can’t conclude [from one case] that it’s becoming more severe in humans.”
Previous iterations of bird flu have had a 50% death rate in humans, Siegel noted, but the 61 human cases in the U.S. this year have been “very mild.”
“That is actually a good sign, that the virus is mutating away from severity, as traditionally H5N1 makes humans very sick,” he told Fox News Digital.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
The most critical thing to watch, according to the doctor, is whether bird flu will begin to spread from human to human.
“That’s the key thing we’re concerned about — that it doesn’t get into the upper respiratory tract among humans,” Siegel said.

“Bird flu is a group of influenza viruses that primarily circulate among bird populations,” an expert told Fox News Digital. “However, influenza viruses are also known to jump species, and bird flu has done this a few times in history.” (Uli Deck/picture alliance via Getty Images)
“It hasn’t up until now, and that’s because it would take some mutations — at least one. We’re tracking that, but probably not closely enough.”
The vast majority of recent human cases have stemmed from direct contact with animals, he said — “but it’s now spread into the cattle population and into milk, which worries a lot of people, including me.”
Rebecca C. Christofferson, PhD, associate professor in the Department of Pathobiological Sciences at Louisiana State University, commented to Fox News Digital about the potential for a pandemic.
“Nobody wants another pandemic.”
“Bird flu is a group of influenza viruses that primarily circulate among bird populations,” she told Fox News Digital.
“However, influenza viruses are also known to jump species, and bird flu has done this a few times in history.”

A researcher wears a protective suit while collecting samples of wildlife where the H5N1 bird flu virus was detected in Chilean Antarctic territory in Antarctica. (Reuters/Instituto Antartico Chileno)
“The more the virus gets into mammals and then the more it passes from mammal to mammal, the greater the concern that it will adapt to mammals and spread more easily among them and then spill over into humans,” Christofferson added.
“Nobody wants another pandemic.”
For more Health articles, visit foxnews.com/health
The good news, Cristofferson said, is that it’s easy to protect yourself from catching bird flu.
“If you have to handle birds or suspected ill animals (or be around ill people), wearing gloves and masks and washing your hands will protect you as it does with other respiratory viruses,” she said.
Health
'Pendulum lifestyle' could be key to juggling daily challenges

For those who are feeling “stuck” or overwhelmed while striving for work-life balance, some experts recommend adopting a “pendulum lifestyle.”
Coined by Dr. Jeffrey Karp, Ph.D, a professor of biomedical engineering at Brigham & Women’s Hospital Harvard Medical School in Boston, the pendulum lifestyle is defined as a “concept that acknowledges life’s natural ebb and flow, and empowers you to thrive amidst the swings.”
“Rarely are we in balance … it’s just unrealistic and an anxiety-inducing expectation,” the doctor told Fox News Digital in an interview.
WHY THERE’S NO SUCH THING AS A ‘WORK-LIFE BALANCE,’ SAYS CAREER COACH AND AUTHOR
Seeing the world as a pendulum fosters a more compassionate mindset and alleviates the pressure to be perfect, Karp said.
For those who are feeling “stuck” or overwhelmed while striving for work-life balance, some experts recommend adopting a “pendulum lifestyle.” (iStock)
With this approach, people can take small steps to “swing the pendulum,” enabling them to feel more emotionally, mentally and physically “balanced” during the day, according to the expert.
This could also empower individuals who feel “stuck” when facing daily challenges, he said.
“Looking at nature, there are so many cycles, so many things that are kind of going back and forth, like night and day …. changes of seasons, and the waxing and waning of the moon,” noted Karp.
MOST PRODUCTIVE THINGS YOU CAN GET DONE EARLY IN THE MORNING BEFORE WORK, ACCORDING TO CAREER COACHES
The pendulum lifestyle involves daily “self-check-ins” where the person gauges their physical, emotional and mental energy levels, Karp said. They can then take immediate steps to move their levels in a positive direction toward the ideal balance.
“If we can visualize everything on a pendulum, we can think, ‘What’s the one step I could take today to bring the pendulum a little closer to where I want it to be?’” he said.
With this approach, people can take small steps to “swing the pendulum,” enabling them to feel more emotionally, mentally and physically “balanced” during the day. (iStock)
For example, a person who has low physical energy could visualize a pendulum with the lowest energy on one side and the highest energy on the other.
He would then do a “self-check” to identify where his energy level lies on the pendulum and what small steps could move it closer to the ideal balance point, Karp said.
“True well-being doesn’t lie in perfection or consistency, but in our ability to navigate the ebb and flow of life.”
That might mean taking a 10-minute walk, doing some jumping jacks or performing a few stretches to move the pendulum to a higher energy level position.
“This empowers the person and reminds them they are not stuck,” Karp said.

Taking a 10-minute walk, doing some jumping jacks or performing a few stretches can move the pendulum to a higher energy level position, the expert said. (iStock)
On the flip side, if it’s late at night and a person needs to wind down, she might engage in a calming exercise like meditation or listening to relaxing music as a way to swing the pendulum to a level more conducive to sleeping, the expert advised.
The pendulum lifestyle can also serve as a mood-booster, Kelp said. When someone is feeling down, watching a funny movie or practicing gratitude can help shift the pendulum.
WHAT IS ‘BRAIN ROT’? THE SCIENCE BEHIND WHAT TOO MUCH SCROLLING DOES TO OUR BRAINS
The approach could also help launch forward momentum if someone feels “stuck” in life, the expert said.
“When you start to realize that you’re not limited to being at that spot on the pendulum, but can take a step forward and be intentional, it’s just so empowering,” he said.

The daily check-in process could help individuals identify when they are feeling in less than tip-top shape and find ways to swing in a better direction, one expert said. (iStock)
Dr. Molly Sherb, an assistant professor of psychiatry at the Icahn School of Medicine at Mount Sinai and a licensed psychologist at Mount Sinai in New York City, commented on Karp’s concept of a pendulum lifestyle.
“When you start to realize that you’re not limited to being at that spot on the pendulum, but can take a step forward and be intentional, it’s just so empowering.”
She agreed that the daily check-in process could help individuals identify when they are feeling in less than tip-top shape and find ways to swing in a better direction.
“That might include getting better sleep or eating a healthier breakfast … to help you wake up with a better bandwidth tomorrow,” Sherb said.
Progress, not perfection
Dr. Christopher Fisher, a psychologist at Zucker Hillside Hospital Northwell Health in Queens, New York, said the pendulum lifestyle could help those who feel pressured to achieve a perfect work-life balance.
“The pendulum of life’s experiences – whether emotional, cognitive or physical – is one of the truest expressions of what it means to be human,” he told Fox News Digital.
TRUMP’S DAYLIGHT SAVING TIME PLAN AND SLEEP: WHAT YOU MUST KNOW
“True well-being doesn’t lie in perfection or consistency, but in our ability to navigate the ebb and flow of life,” he told Fox News Digital.
Sherb agreed that the essence of the pendulum lifestyle is that it’s not always possible to strike that 50-50 equal balance.

Adopt a constructive viewpoint and ask yourself what positive changes or routines can help you achieve a more optimal level on the pendulum path, one expert advised. (iStock)
“It’s about constantly tuning into yourself … and seeing which parts of your life might need you more at certain times,” she said.
“It’s not a one-size-fits-all approach, but a more tailored approach based on what you need and what people in your life need from you.”
4 steps to implementing the pendulum lifestyle
Karp shared some specific strategies for adopting the pendulum approach.
1. Perform a head-to-toe check-in each morning
Ask yourself how you are feeling emotionally, physically and mentally. What parts do not feel at a 100% level?
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
2. Make any necessary adjustments
Based on your self-check-in, consider changing your routine to accommodate your energy level or take simple steps to help move the pendulum in a positive direction, Karp suggested.
3. Be compassionate and curious
If you feel off-balance, Karp said to recognize that as part of the natural pendulum swing and to embrace it with self-compassion rather than shame and criticism.
“It’s about constantly tuning into yourself … and seeing which parts of your life might need you more at certain times.”
Adopt a constructive viewpoint and ask yourself what positive changes or routines can help you achieve a more optimal level on the pendulum path, he advised.
4. Understand your pendulum swings
It could be helpful to ask yourself specific questions, such as the following.
“What factors helped contribute to a state of feeling balanced?”
“What factors contributed to feeling off-balance?”
“What small changes can I make today to foster a sense of better balance?”
“How did I respond to feeling imbalanced and was it effective?”
For more Health articles, visit www.foxnews.com/health
Above all, Karp said, it’s important to remember that finding balance is a “lifelong journey.”
-

 Politics1 week ago
Politics1 week agoCanadian premier threatens to cut off energy imports to US if Trump imposes tariff on country
-
/cdn.vox-cdn.com/uploads/chorus_asset/file/25782636/247422_ChatGPT_anniversary_CVirginia.jpg)
/cdn.vox-cdn.com/uploads/chorus_asset/file/25782636/247422_ChatGPT_anniversary_CVirginia.jpg) Technology1 week ago
Technology1 week agoInside the launch — and future — of ChatGPT
-
/cdn.vox-cdn.com/uploads/chorus_asset/file/25789444/1258459915.jpg)
/cdn.vox-cdn.com/uploads/chorus_asset/file/25789444/1258459915.jpg) Technology1 week ago
Technology1 week agoOpenAI cofounder Ilya Sutskever says the way AI is built is about to change
-

 Politics1 week ago
Politics1 week agoU.S. Supreme Court will decide if oil industry may sue to block California's zero-emissions goal
-
/cdn.vox-cdn.com/uploads/chorus_asset/file/25546252/STK169_Mark_Zuckerburg_CVIRGINIA_D.jpg)
/cdn.vox-cdn.com/uploads/chorus_asset/file/25546252/STK169_Mark_Zuckerburg_CVIRGINIA_D.jpg) Technology1 week ago
Technology1 week agoMeta asks the US government to block OpenAI’s switch to a for-profit
-

 Politics1 week ago
Politics1 week agoConservative group debuts major ad buy in key senators' states as 'soft appeal' for Hegseth, Gabbard, Patel
-

 Business6 days ago
Business6 days agoFreddie Freeman's World Series walk-off grand slam baseball sells at auction for $1.56 million
-
/cdn.vox-cdn.com/uploads/chorus_asset/file/23951353/STK043_VRG_Illo_N_Barclay_3_Meta.jpg)
/cdn.vox-cdn.com/uploads/chorus_asset/file/23951353/STK043_VRG_Illo_N_Barclay_3_Meta.jpg) Technology6 days ago
Technology6 days agoMeta’s Instagram boss: who posted something matters more in the AI age















