Health
Alzheimer’s experimental drug may slow progression of disease, but there are risks: experts
The experimental drug lecanemab has indicated a slowing of cognitive decline development by 27% in sufferers with early-stage Alzheimer’s, based on a examine revealed this week within the New England Journal of Medication.
“These findings present that lecanemab gives promise for folks with early Alzheimer’s illness, with a major slowing of decline and an inexpensive security profile,” the examine’s lead researcher, Dr. Christopher H. Van Dyck, informed Fox Information Digital in an interview.
Van Dyck is director of the Alzheimer’s Illness Analysis Unit and a professor of psychiatry, neurology and neuroscience on the Yale College College of Medication.
ALZHEIMER’S DRUG STUDY YIELDS POSITIVE RESULTS IN EARLY STAGES OF DISEASE
Within the examine, Van Dyck’s researchers stated the drug lecanemab “diminished markers of amyloid in early Alzheimer’s illness and resulted in reasonably much less decline on measures of cognition and performance than placebo at 18 months — however was related to antagonistic occasions.”
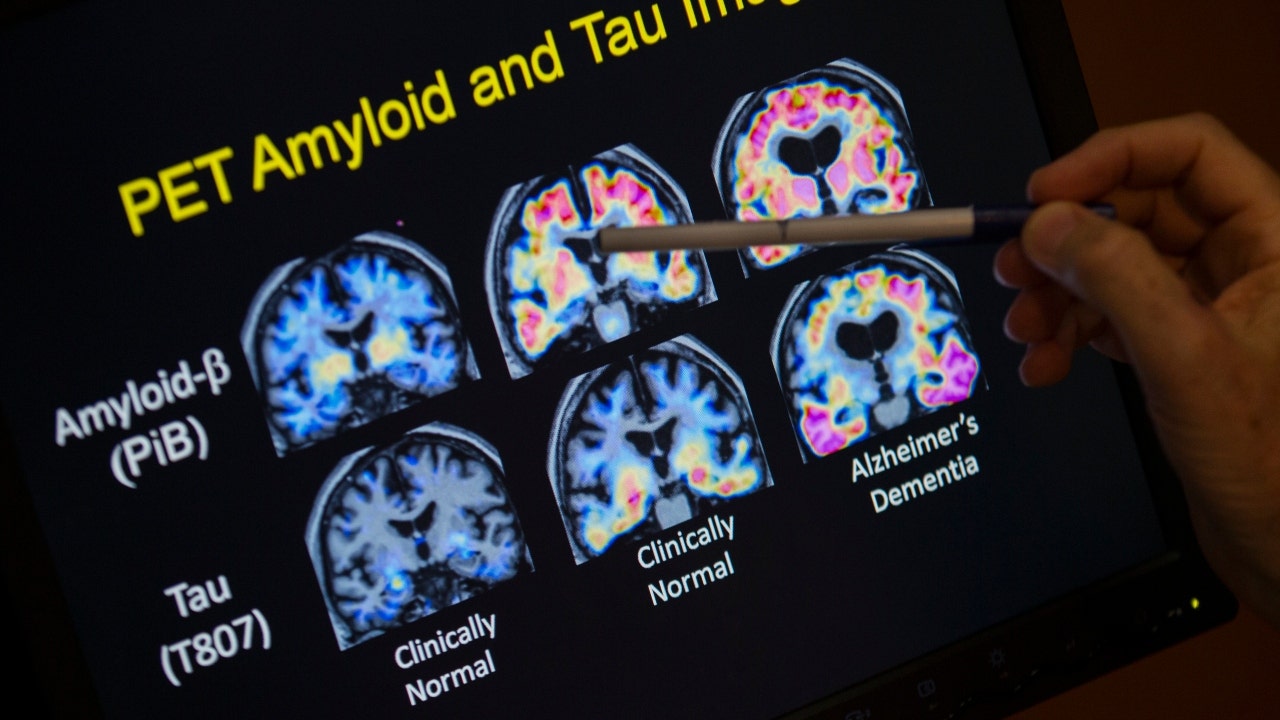
On this Might 19, 2015, file picture, a health care provider factors to PET scan outcomes which are a part of a examine on Alzheimer’s illness at a hospital in Washington. Scientists know that lengthy earlier than the reminiscence issues of Alzheimer’s turn out to be apparent, folks expertise extra delicate modifications of their considering and judgment.
(AP Picture/Evan Vucci)
Pharmaceutical corporations Eisai Co. Ltd. and Biogen Inc., developed the drug.
The drug is a monoclonal antibody — it interferes with the formation of amyloid plaque, thought-about a significant offender in Alzheimer’s illness, based on specialists.
‘Related to much less medical decline’
The examine included 1,795 members who had early Alzheimer’s illness.
Of the members, 898 acquired lecanemab, whereas 897 acquired a placebo in 235 websites positioned in North America, Asia and Europe from March 2019 to March 2021, based on the examine.
NOSE PICKING WAS ALWAYS GROSS — NOW A STUDY SAYS IT MAY LEAD TO LATE-ONSET ALZHEIMER’S
The members got lecanemab intravenously each two weeks over an 18-month interval.
“Analyses at 18 months confirmed dose- and time-dependent clearance of amyloid with lecanemab, and the drug was related to much less medical decline on some measures than placebo,” the researchers stated within the revealed examine.
The lecanemab group mirrored slower development in cognitive decline.
The investigators relied on a software that measures cognitive impairment. It is referred to as the Medical Dementia Score-Sum of Packing containers (CDR-SB) — it follows the members’ progress.
The common baseline scores had been roughly 3.2 in each the lecanemab and placebo teams. A rating of 0.5 to six is in line with early Alzheimer’s illness, based on the researchers.

The headquarters of Biogen Inc. are proven in March 2020 in Cambridge, Massachusetts. Along with Eisai Co. Ltd., Biogen developed the brand new drug.
(AP Picture/Steven Senne, File)
After 18 months of remedy, the typical rating of the lecanemab group went up by 1.21 factors, in comparison with sufferers within the placebo group — who noticed their rating go up by 1.66 factors.
The lecanemab group mirrored slower development in cognitive decline.
Some members did expertise antagonistic occasions, the researchers famous.
NEW STUDY INDICATES DEMENTIA SIGNS CAN BE DETECTED NEARLY A DECADE BEFORE DIAGNOSIS
Roughly 20% of sufferers receiving lecanemab skilled mind swelling or mind bleeding, based on the examine.
Lecanemab is up for FDA approval in early 2023.
Eisai stated two deaths occurred, although they weren’t thought-about linked to lecanemab.
Officers on the Alzheimer’s Discovery Drug Basis (ADDF) stated in a launched assertion that lecanemab, which is up for FDA approval in early 2023, represents a optimistic step towards remedy of the illness and “welcomed information for the thousands and thousands of sufferers and households residing with Alzheimer’s.”
![Said one leading expert in Alzheimer's about the new findings, "We have a lot of ground to cover to get from the 27% slowing [that] lecanemab offers to our goal of slowing cognitive decline by 100%."](https://a57.foxnews.com/static.foxnews.com/foxnews.com/content/uploads/2020/12/640/320/iStock-648706624.jpg?ve=1&tl=1)
Stated one main knowledgeable in Alzheimer’s in regards to the new findings, “We’ve a whole lot of floor to cowl to get from the 27% slowing [that] lecanemab gives to our aim of slowing cognitive decline by 100%.”
(iStock)
Dr. Howard Fillit, co-founder and chief science officer on the ADDF, additionally stated within the assertion, “However that is solely a begin to stopping Alzheimer’s in its tracks. We’ve a whole lot of floor to cowl to get from the 27% slowing [that] lecanemab gives to our aim of slowing cognitive decline by 100%.”
The ADDF assertion stated amyloid-clearing medicine are a part of the answer in addressing Alzheimer’s illness.
COGNITIVE DECLINE CAN BE AVOIDED WITH SIMPLE, EVERYDAY EXERCISES, NEW STUDY SUGGESTS
But additional improvement is required of a brand new era of medicines that may goal particular pathologies that contribute to the illness.
The Alzheimer’s Affiliation stated it was inspired by the worldwide medical trial of lecanemab.
“Distinctive drug combos matched to every affected person’s underlying pathologies is the reply and our greatest hope to offer sufferers long-lasting reduction from this insidious and progressive illness,” Fillit stated within the launched assertion.
The Alzheimer’s Affiliation additionally launched a press release concerning the part three-trial outcomes.
One knowledgeable who was not concerned within the examine informed Fox Information Digital she was excited to see the statistically vital distinction between the lecanemab and placebo teams within the examine — however cautioned that extra analysis on the Alzheimer’s drug is required.
(iStock)
On its web site, the group stated it was inspired by the worldwide medical trial of lecanemab.
It stated the examine “confirms this remedy can meaningfully change the course of the illness for folks within the earliest levels of Alzheimer’s illness. The Alzheimer’s Affiliation requires the Meals and Drug Administration’s accelerated approval of lecanemab.”
The group additionally famous, partially, “These peer-reviewed, revealed outcomes present lecanemab will present sufferers extra time to take part in each day life and dwell independently. It may imply many months extra of recognizing their partner, kids and grandchildren.”
“Statistically vital doesn’t all the time imply virtually vital, particularly not within the setting of great dangers.”
Dr. Marzena Gieniusz, medical program director of the Alzheimer’s and Dementia Care (ADC) Program at Northwell Well being on Lengthy Island, New York, commented on the findings.
Dr. Gieniusz, who was not concerned within the examine, stated she was excited to see the statistically vital distinction between the lecanemab and placebo teams within the examine — however cautioned that extra analysis on the drug is required.
“Statistically vital doesn’t all the time imply virtually vital, particularly not within the setting of great dangers, which had been famous within the examine, in addition to the dangers not but evident — together with the potential for elevated hospitalizations, pointless interventions, and so on.”

“The FDA is predicted to determine whether or not to grant accelerated approval to lecanemab by January 6, 2023,” stated the Alzheimer’s Affiliation.
(iStock)
Gieniusz additionally informed Fox Information Digital, “Though I’m glad to see the outcomes to date, I’m wanting to be taught extra, together with in regards to the security and efficacy, earlier than meaningfully exploring and contemplating the sensible dangers, advantages and options of this drug.”
DRINKING COFFEE, TEA CAN LOWER THE RISK OF DEMENTIA, STROKE: STUDY
Van Dyck of the Alzheimer’s Illness Analysis Unit informed Fox Information Digital that additional analysis is presently underway — and that researchers want members.
“The subsequent steps in our analysis of this remedy will definitely be to go nonetheless earlier to asymptomatic, at-risk people.” (The trial in preclinical Alzheimer’s illness has been underway since 2019, however is behind in enrollment.)
Van Dyck stated he’s “optimistic” that the “outcomes will spur curiosity and enrollment and permit us to finish that essential examine.”
Van Dyck additionally stated he’s “optimistic” that the “outcomes will spur curiosity and enrollment and permit us to finish that essential examine. Along with the essential results in early symptomatic AD, we wish to know if that may be considerably enlarged by treating people earlier than a lot injury happens and vital signs start.”
Additionally, based on the Alzheimer’s Affiliation, there is a potential pricey drawback as a result of a Facilities for Medicare and Medicaid Companies (CMS) coverage that might block entry to the remedy if the FDA approves it.
“The FDA is predicted to determine whether or not to grant accelerated approval to lecanemab by January 6, 2023,” the affiliation stated.
“Ought to the FDA achieve this, the present CMS coverage will stop 1000’s and 1000’s of Medicare beneficiaries with a terminal, progressive illness from accessing this remedy throughout the restricted span of time they should entry it.”
The affiliation stated CMS pledged to maneuver shortly to change the coverage if new proof was introduced.
Now, given the brand new proof, “CMS can start its evaluation instantly,” the related stated. “The Alzheimer’s Affiliation calls on CMS to revise its coverage with the utmost urgency.”

Health
Nutrition Pro's Easy Sugar Detox Breaks the Cravings Cycle to Boost Weight Loss — Here's How to Do It

Sign Up
Create a free account to access exclusive content, play games, solve puzzles, test your pop-culture knowledge and receive special offers.
Already have an account? Login
Forgot your password?
Get back to the Sign In
Use left and right arrow keys to navigate between menu items.
Use escape to exit the menu.
Health
Having trouble sleeping? It could be for this surprising reason, experts say

When creating an ideal sleeping environment, you might think of lighting, temperature and sound — but what about food?
What you eat during the day can have a surprising impact on how well you sleep at night, according to experts.
“Food choice is an essential consideration for ensuring good sleep quality. Some types of food promote sleep while others may cause sleep disruption,” Dr. Chelsie Rohrscheib, head sleep expert at Wesper, a sleep analysis company in New York, told Fox News Digital.
LACK OF SLEEP COULD BE A FACTOR IN A ‘SILENT EPIDEMIC,’ EXPERTS WARN
Here’s what to know.
Signs that food is interfering with sleep
If after eating you’re struggling to fall asleep, waking up often during the night or experiencing heartburn, acid reflux or indigestion, your food choices could be the culprit, according to Dr. Raj Dasgupta, chief medical adviser at Sleepopolis in California.
What you eat can have an impact on how well you sleep at night, experts say. (iStock)
Other warning signs include experiencing restlessness or stomach discomfort, needing more frequent bathroom breaks at night, or waking up feeling groggy or unrested.
“Having intense dreams or nightmares or noticing changes in your usual sleep routine” are other indications that food could be interfering with sleep, Dasgupta said.
“Paying attention to these cues can help you figure out if certain foods or drinks are messing with your sleep quality, so you can make adjustments as needed for better rest,” he said.
Best foods to eat for quality sleep
Foods that encourage better sleep include meals with a good amount of lean protein, meals that are high in fiber, and meals that are rich in complex carbohydrates, according to Rohrscheib.
“This food combination keeps us feeling full and satisfied throughout the night and prevents us from waking up from hunger,” she said.

“Having intense dreams or nightmares or noticing changes in your usual sleep routine” are other indications that food choices could be interfering with sleep, an expert said. (iStock)
“It also reduces the risk of indigestion and heartburn.”
Foods containing dairy are especially beneficial, she said, because they contain tryptophan, an amino acid that is essential for the production of serotonin and melatonin, two chemicals needed for sleep.
“Food choice is an essential consideration for ensuring good sleep quality.”
Bananas can also help promote sleep, according to Dasgupta.
“They contain magnesium and tryptophan, which can help you relax and boost production of sleep-inducing hormones,” he told Fox News Digital.

Almonds provide magnesium for muscle relaxation and also contain protein and healthy fats to keep blood sugar levels stable, said an expert. (iStock)
Almonds also provide magnesium for muscle relaxation; they contain protein and healthy fats to keep blood sugar levels stable, he said.
“Cherries contain natural melatonin, potentially helping to regulate your sleep-wake cycles,” Dasgupta said.
IMPROVE YOUR SLEEP BY OPTIMIZING 6 BIOMARKERS: ‘INTEGRAL TO HEALTH’
Oatmeal is also a sleep-friendly food.
“Its complex carbohydrates increase serotonin levels, while its melatonin content helps to regulate sleep,” said Dasgupta.
As we all hear around Thanksgiving time, turkey is rich in tryptophan, facilitating the production of serotonin and melatonin, Dasgupta noted.

Bananas can help with quality sleep because they contain magnesium and tryptophan, which can promote relaxation, an expert said. (iStock)
“Kiwi is loaded with antioxidants, vitamins and serotonin, all of which support sleep pattern regulation,” he said.
Dasgupta also recommends eating Greek yogurt to promote improved sleep, as its calcium content assists in the body’s use of tryptophan for melatonin production, while its protein helps maintain blood sugar levels.
6 SURPRISINGLY SIMPLE WAYS TO KEEP YOURSELF HEALTHY (HINT: SLEEP IS INVOLVED)
“Finally, warm milk, with its tryptophan content and comforting warmth, can help you relax” for a good night’s sleep, he said.
Those who are lactose-intolerant can opt for warm almond milk.
Foods that can disrupt sleep
Some foods are more likely to cause indigestion and heartburn, which makes it difficult to fall asleep and maintain sleep, according to Rohrscheib.
“This includes foods with high fat or acid content, foods containing caffeine, or spicy foods,” she said.
Dasgupta agreed that eating heavy or spicy foods ahead of bedtime can cause stomach discomfort, heartburn and acid reflux, which can make it harder to settle down comfortably.

Eating heavy or spicy foods can cause stomach discomfort, heartburn and acid reflux, which can make it harder to settle down comfortably for the night, an expert said. (iStock)
“Greasy or heavy meals take longer to digest, which can leave you feeling uncomfortable and disrupt your sleep,” he advised.
Caffeine is also a common culprit in sleep disruption — experts recommend avoiding it in the hours leading up to bedtime.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
“Any food containing caffeine, even small amounts, should be avoided to prevent sleep disruption,” Rohrscheib said. “This includes coffee, some teas, sodas, energy drinks and some chocolates.”
It’s best to abstain from alcohol as well, Dasgupta said. “While it might seem like a nightcap, it messes with your sleep cycles, leading to worse sleep quality.”

Caffeine is also a common culprit in sleep disruption — experts recommend avoiding it in the hours leading up to bedtime. (iStock)
Highly processed foods and foods containing high amounts of sugar cause a quick spike in glucose levels and increase the risk of a “blood sugar crash,” also known as hypoglycemia, Rohrscheib warned.
“When we’re hypoglycemic, our brain will attempt to wake us up to eat more food to normalize our blood glucose levels,” she said. “Thus, these foods should be avoided before bedtime.”
“Consuming too much and making yourself over-full is likely to make you uncomfortable and cause poor sleep quality.”
“Lastly, processed or junk foods, loaded with additives and unhealthy fats, can throw off your sleep patterns,” Dasgupta added.
Portion size is also a factor in sleep quality, both experts agreed.
“Regardless of the type of meal you eat, consuming too much and making yourself over-full is likely to make you uncomfortable and cause poor sleep quality,” Rohrscheib said.
For more Health articles, visit www.foxnews.com/health.
Health
World Health Organization, experts reach landmark agreement on how to define airborne diseases

- The World Health Organization and 500 experts have established a consensus on the definition of airborne diseases.
- The groups are aiming to prevent confusion similar to that experienced during the early stages of the COVID-19 pandemic.
- The WHO released a document outlining the definition, marking a step toward improving prevention of diseases transmitted through the air.
The World Health Organization and around 500 experts have agreed for the first time what it means for a disease to spread through the air, in a bid to avoid the confusion early in the COVID-19 pandemic that some scientists have said cost lives.
The Geneva-based U.N. health agency released a technical document on the topic on Thursday. It said it was the first step towards working out how to better prevent this kind of transmission, both for existing diseases like measles and for future pandemic threats.
The document concludes that the descriptor “through the air” can be used for infectious diseases where the main type of transmission involves the pathogen traveling through the air or being suspended in the air, in line with other terms such as “waterborne” diseases, which are understood across disciplines and by the public.
WHO DIRECTOR CALLS FOR WORLD PANDEMIC TREATY TO PREPARE FOR DISEASE X
Almost 500 experts contributed to the definition, including physicists, public health professionals and engineers, many of whom disagreed bitterly over the topic in the past.
The World Health Organization and around 500 experts have agreed for the first time what it means for a disease to spread through the air, in a bid to avoid the confusion early in the COVID-19 pandemic that some scientists have said cost lives. (REUTERS/Denis Balibouse/File Photo)
Agencies have historically required high levels of proof before calling diseases airborne, which required very stringent containment measures; the new definition says the risk of exposure and severity of disease should also be considered.
Past disagreements also centered around whether infectious particles were “droplets” or “aerosols” based on size, which the new definition moves away from.
During the early days of COVID in 2020, around 200 aerosol scientists publicly complained that the WHO had failed to warn people of the risk that the virus could spread through the air. This led to an overemphasis on measures like handwashing to stop the virus, rather than focusing on ventilation, they said.
By July 2020, the agency said there was “evidence emerging” of airborne spread, but its then chief scientist Soumya Swaminathan – who began the process to get a definition – later said the WHO should have been more forceful “much earlier”.
Her successor, Jeremy Farrar, said in an interview that the new definition was about more than COVID, but he added that at the beginning of the pandemic there was a lack of evidence available and experts including the WHO acted in “good faith”. At that time, he was head of the Wellcome Trust charity and advised the British government on the pandemic.
Farrar said getting the definition agreed among experts from all disciplines would allow discussions to begin about issues such as ventilation in many different settings, from hospitals to schools.
He compared it to the realization that blood-borne viruses like HIV or hepatitis B could be spread by medics not wearing gloves during procedures.
“When I started out, medical students, nurses, doctors, none of us wore gloves to take blood,” he told Reuters. “Now it is unthinkable that you wouldn’t wear gloves. But that came because everyone agreed on what the issue was, they agreed on the terminology… [The change in practice] came later.”
-

 News1 week ago
News1 week agoVideo: Election Officials Continue To Face Violent Threats
-

 World1 week ago
World1 week agoHope and anger in Gaza as talks to stop Israel’s war reconvene
-
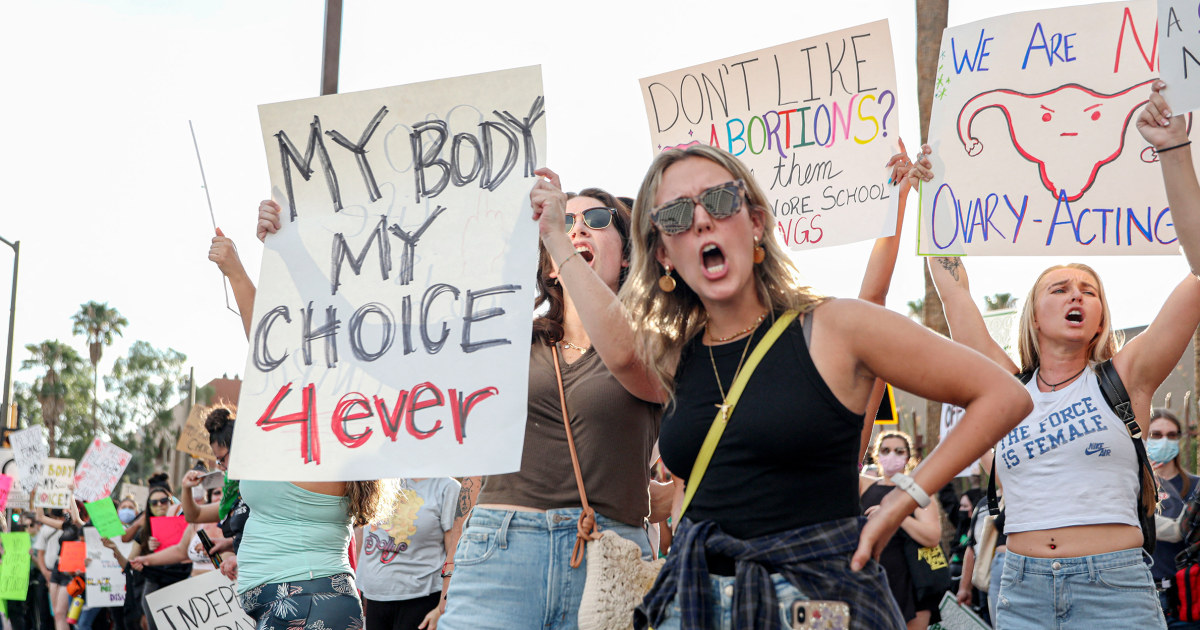 News1 week ago
News1 week agoArizona Supreme Court rules that a near-total abortion ban from 1864 is enforceable
-

 Midwest1 week ago
Midwest1 week agoFormer Chicago Mayor Lori Lightfoot hired to investigate so-called 'worst mayor in America' at $400 an hour
-
/cdn.vox-cdn.com/uploads/chorus_asset/file/25382021/V4_Pro_Beta_PressKit_LaunchImage.jpg)
/cdn.vox-cdn.com/uploads/chorus_asset/file/25382021/V4_Pro_Beta_PressKit_LaunchImage.jpg) Technology1 week ago
Technology1 week agoAdobe overhauls Frame.io to make it a little more Trello-like
-

 Movie Reviews1 week ago
Movie Reviews1 week agoThe Long Game (2024) – Movie Review
-

 World1 week ago
World1 week agoEU migration reform faces tight vote as party divisions deepen
-

 Politics1 week ago
Politics1 week agoBillionaire who helped Trump with $175M bond says he 'probably didn't charge enough'